Page 362 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 362
298 Part IV Disorders of Hematopoietic Cell Development
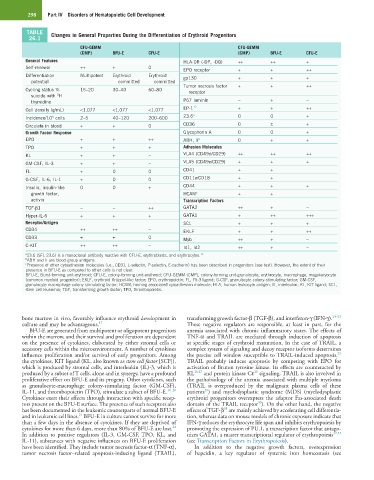
TABLE Changes in General Properties During the Differentiation of Erythroid Progenitors
26.1
CFU-GEMM CFU-GEMM
(CMP) BFU-E CFU-E (CMP) BFU-E CFU-E
General Features HLA-DR (-DP, -DQ) ++ ++ +
Self-renewal ++ + 0 EPO receptor + + ++
Differentiation Multipotent Erythroid Erythroid gp130 + + +
potential committed committed
Tumor necrosis factor + + ++
Cycling status % 15–20 30–40 60–80 receptor
3
suicide with H
thymidine P67 laminin − + −
Cell density (g/mL) <1.077 <1.077 <1.077 EP-1 12 + + ++
Incidence/10 cells 2–5 40–120 200–600 23.6 a 0 0 +
5
Circulate in blood + + 0 CD36 0 ± +
Growth Factor Response Glycophorin A 0 0 +
EPO + + ++ ABH, Ii b 0 + +
TPO + + + Adhesion Molecules
KL + + − VLA4 (CD49d/CD29) ++ ++ ++
GM-CSF, IL-3 + + − VLA5 (CD49e/CD29) + + +
FL + 0 0 CD41 + +
G-CSF, IL-6, IL-1 + 0 0 CD11a/CD18 + +
Insulin, insulin-like 0 0 + CD44 + + +
growth factor, HCAM c + +
activin Transcription Factors
TGF-β1 − − ++ GATA2 ++ + −
Hyper-IL-6 + + + GATA1 + ++ +++
Receptor/Antigen SCL + + +
CD34 ++ ++ − EKLF + + ++
CD33 + + 0 Myb ++ + −
C-KIT ++ ++ − Id1, Id2 ++ + −
a 23.6 (SFL 23.6) is a monoclonal antibody reactive with CFU-E, erythroblasts, and erythrocytes. 13
b ABH and Ii are blood group antigens.
c Presence of other cytoadhesion molecules (i.e., CD31, L-selectin, P-selectin, E-cadherin) has been described in progenitors (see text). However, the extent of their
presence in BFU-E as compared to other cells is not clear.
BFU-E, Burst-forming unit-erythroid; CFU-E, colony-forming unit-erythroid; CFU-GEMM (CMP), colony-forming unit-granulocyte, erythrocyte, macrophage, megakaryocyte
(common myeloid progenitor); EKLF, erythroid Krüppel-like factor; EPO, erythropoietin; FL, Flt-3 ligand; G-CSF, granulocyte colony-stimulating factor; GM-CSF,
granulocyte-macrophage colony-stimulating factor; HCAM, homing-associated cytoadhesion molecule; HLA, human leukocyte antigen; IL, interleukin; KL, KIT ligand; SCL,
stem cell leukemia; TGF, transforming growth factor; TPO, thrombopoietin.
bone marrow in vivo, favorably influence erythroid development in transforming growth factor-β (TGF-β), and interferon-γ (IFN-γ). 23–25
culture and may be advantageous. 17 These negative regulators are responsible, at least in part, for the
BFU-E are generated from multipotent or oligopotent progenitors anemia associated with chronic inflammatory states. The effects of
within the marrow, and their survival and proliferation are dependent TNF-α and TRAIL are mediated through induction of apoptosis
on the presence of cytokines, elaborated by either stromal cells or at specific stages of erythroid maturation. In the case of TRAIL, a
accessory cells within the microenvironment. A number of cytokines complex system of signaling and decoy receptor isoforms determines
25
influence proliferation and/or survival of early progenitors. Among the precise cell window susceptible to TRAIL-induced apoptosis.
the cytokines, KIT ligand (KL, also known as stem cell factor [SCF]), TRAIL probably induces apoptosis by competing with EPO for
which is produced by stromal cells, and interleukin (IL)-3, which is activation of Bruton tyrosine kinase. Its effects are counteracted by
28
produced by a subset of T cells, alone and in synergy, have a profound KL 26,27 and protein kinase Cε signaling. TRAIL is also involved in
proliferative effect on BFU-E and its progeny. Other cytokines, such the pathobiology of the anemia associated with multiple myeloma
as granulocyte-macrophage colony-stimulating factor (GM-CSF), (TRAIL is overproduced by the malignant plasma cells of these
29
IL-11, and thrombopoietin (TPO), stimulate a subset of BFU-E. 18–20 patients ) and myelodysplastic syndrome (MDS) (myelodysplastic
Cytokines exert their effects through interaction with specific recep- erythroid progenitors overexpress the adaptor Fas-associated death
30
tors present on the BFU-E surface. The presence of such receptors also domain of the TRAIL receptor ). On the other hand, the negative
31
has been documented in the leukemic counterparts of normal BFU-E effects of TGF-β are mainly achieved by accelerating cell differentia-
21
and in leukemic cell lines. BFU-E in culture cannot survive for more tion, whereas data on mouse models of chronic exposure indicate that
than a few days in the absence of cytokines. If they are deprived of IFN-γ reduces the erythrocyte life span and inhibits erythropoiesis by
22
cytokines for more than 6 days, more than 80% of BFU-E are lost. promoting the expression of PU.1, a transcription factor that antago-
In addition to positive regulators (IL-3, GM-CSF, TPO, KL, and nizes GATA1, a master transcriptional regulator of erythropoiesis 32,33
IL-11), substances with negative influences on BFU-E proliferation (see Transcription Factors in Erythropoiesis).
have been identified. They include tumor necrosis factor-α (TNF-α), In addition to the negative growth factors, overexpression
tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), of hepcidin, a key regulator of systemic iron homeostasis (see