Page 655 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 655
Chapter 40 Thalassemia Syndromes 557
approximately 200 g/mol. It is an orally active bidentate iron chelator
that requires three molecules to bind one iron atom. Deferiprone is 1.0
absorbed by the gastrointestinal tract and has a plasma half-life of
approximately 90 minutes (2–3 hours). Chelated iron is excreted 0.9
predominantly in the urine (90%) and far less in the stool (10%). It 0.8
was synthesized in the late 1980s and was first tested in uncontrolled
clinical trials at the Royal Free Hospital in London, hence the eponym 0.7
L1. 205,206 A large observational study demonstrated improvement in 0.6
207
cardiac iron deposition with deferiprone treatment. At doses of Cumulative proportion adverse reaction free 0.5
75 mg/kg/d, deferiprone administered in three divided doses with
meals reduces or maintains iron stores, thereby achieving negative 0.4 Complete Censored
iron balance or iron balance in many regularly transfused patients for 0.3 Agranulocytosis: 1/187 = 0.5%
the most part, particularly those with more severe transfusional iron Neutropenia: 15/187 = 8.0%
overload. 208–216 However, some patients remain in positive iron 0.2 Arthropathy: 28/187 = 15.0%
balance and continue to accumulate iron during long-term therapy 0.1 GI symptoms: 62/187 = 33.2%
with this dose of deferiprone. 209,212,217 Regimens using higher doses
218
up to 100 mg/kg/d of deferiprone may be more effective. Combi- 0.0
nation regimens with deferoxamine also reduce iron stores or prove 0 4 8 12 16 20 24 28 32 36 40 44 48 52
effective in restoring negative iron balance in some of these Time (mo)
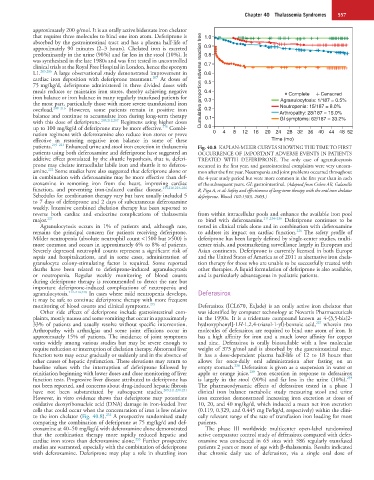
patients. 219–221 Enhanced urine and stool iron excretion in thalassemia Fig. 40.8 KAPLAN-MEIER CURVES SHOWING THE TIME TO FIRST
patients using both deferoxamine and deferiprone have suggested an OCCURRENCE OF IMPORTANT ADVERSE EVENTS IN PATIENTS
additive effect postulated by the shuttle hypothesis, that is, deferi- TREATED WITH DEFERIPRONE. The only case of agranulocytosis
prone may chelate intracellular labile iron and shuttle it to deferox- occurred in the first year, and gastrointestinal complaints were very uncom-
222
amine. Some studies have also suggested that deferiprone alone or mon after the first year. Neutropenia and joint problems occurred throughout
in combination with deferoxamine may be more effective than def- the 4-year study period but were more common in the first year than in each
eroxamine in removing iron from the heart, improving cardiac of the subsequent years. GI: gastrointestinal. (Adapted from Cohen AR, Galanello
function, and preventing iron-induced cardiac disease. 133,216,223–226 R, Piga A, et al: Safety and effectiveness of long-term therapy with the oral iron chelator
Schedules for combination therapy vary but have usually included 5 deferiprone. Blood 102:1583, 2003.)
to 7 days of deferiprone and 2 days of subcutaneous deferoxamine
weekly. Intensive combined chelation therapy has been reported to
reverse both cardiac and endocrine complications of thalassemia from within intracellular pools and enhance the available iron pool
major. 227 to bind with deferoxamine. 211,234–236 Deferiprone continues to be
Agranulocytosis occurs in 1% of patients and, although rare, tested in clinical trials alone and in combination with deferoxamine
226
remains the principal concern for patients receiving deferiprone. to address its impact on cardiac function. The safety profile of
Milder neutropenia (absolute neutrophil count <1500 but >500) is deferiprone has been largely defined by single-center studies, multi-
more common and occurs in approximately 6% to 8% of patients. center trials, and postmarketing surveillance largely in European and
Severely depressed neutrophil counts represent a significant risk of Asian continents. Deferiprone is currently licensed in both Europe
sepsis and hospitalizations, and in some cases, administration of and the United States of America as of 2011 as alternative iron chela-
granulocyte colony-stimulating factor is required. Some reported tion therapy for those who are unable to be successfully treated with
deaths have been related to deferiprone-induced agranulocytosis other therapies. A liquid formulation of deferiprone is also available,
or neutropenia. Regular weekly monitoring of blood counts and is particularly advantageous in pediatric patients.
during deferiprone therapy is recommended to detect the rare but
important deferiprone-induced complications of neutropenia and
agranulocytosis. 212–214,228 In cases where mild neutropenia develops, Deferasirox
it may be safe to continue deferiprone therapy with more frequent
monitoring of blood counts and clinical symptoms. 229 Deferasirox (ICL670, ExJade) is an orally active iron chelator that
Other side effects of deferiprone include gastrointestinal com- was identified by computer technology at Novartis Pharmaceuticals
plaints, mostly nausea and some vomiting that occur in approximately in the 1990s. It is a tridentate compound known as 4-(3,5-bis[2-
237
33% of patients and usually resolve without specific intervention. hydroxyphenyl]-1H-1,2,4-triazol-1-yl)-benzoic acid, wherein two
Arthropathy with arthralgias and some joint effusions occur in molecules of deferasirox are required to bind one atom of iron. It
approximately 15% of patients. The incidence of joint symptoms has a high affinity for iron and a much lower affinity for copper
varies widely among various studies but may be severe enough to and zinc. Deferasirox is orally bioavailable with a low molecular
require reduction or interruption of chelation therapy. Abnormal liver weight of 373 g/mol and is absorbed by the gastrointestinal tract.
function tests may occur gradually or suddenly and in the absence of It has a dose-dependent plasma half-life of 12 to 18 hours that
other causes of hepatic dysfunction. These elevations may return to allows for once-daily oral administration after fasting on an
238
baseline values with the interruption of deferiprone followed by empty stomach. Deferasirox is given as a suspension in water or
239
reinitiation beginning with lower doses and close monitoring of liver apple or orange juice. Iron excretion in response to deferasirox
239
function tests. Progressive liver disease attributed to deferiprone has is largely in the stool (90%) and far less in the urine (10%).
not been reported, and concerns about drug-induced hepatic fibrosis The pharmacodynamic effects of deferasirox tested in a phase I
have not been substantiated by subsequent studies. 209,211,230,231 clinical iron balance metabolic study measuring stool and urine
However, in vitro evidence shows that deferiprone may potentiate iron excretion demonstrated increasing iron excretion at doses of
oxidative deoxyribonucleic acid (DNA) damage in iron-loaded liver 10, 20, and 40 mg/kg/d, which induced a mean net iron excretion
cells that could occur when the concentration of iron is low relative (0.119, 0.329, and 0.445 mg Fe/kg/d, respectively) within the clini-
232
to the iron chelator (Fig. 40.8). A prospective randomized study cally relevant range of the rate of transfusion iron loading for most
comparing the combination of deferiprone at 75 mg/kg/d and def- patients.
eroxamine at 40–50 mg/kg/d with deferoxamine alone demonstrated The phase III worldwide multicenter open-label randomized
that the combination therapy more rapidly reduced hepatic and active comparator control study of deferasirox compared with defer-
233
cardiac iron stores than deferoxamine alone. Further prospective oxamine was conducted in 65 sites with 586 regularly transfused
studies are warranted, especially with the combination of deferiprone patients 2 years or more of age with β-thalassemia. Results indicated
with deferoxamine. Deferiprone may play a role in shuttling iron that chronic daily use of deferasirox, via a single oral dose of