Page 1583 - Williams Hematology ( PDFDrive )
P. 1583
1558 Part XI: Malignant Lymphoid Diseases Chapter 93: Hairy Cell Leukemia 1559
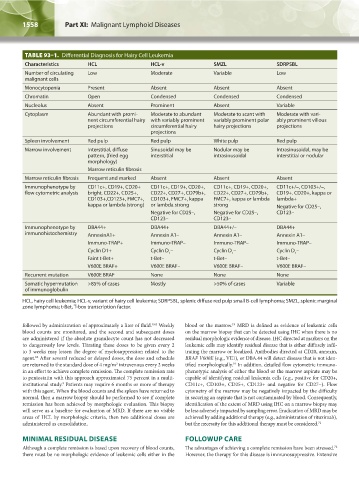
TABLE 93–1. Differential Diagnosis for Hairy Cell Leukemia
Characteristics HCL HCL-v SMZL SDRPSBL
Number of circulating Low Moderate Variable Low
malignant cells
Monocytopenia Present Absent Absent Absent
Chromatin Open Condensed Condensed Condensed
Nucleolus Absent Prominent Absent Variable
Cytoplasm Abundant with promi- Moderate to abundant Moderate to scant with Moderate with vari-
nent circumferential hairy with variably prominent variably prominent polar ably prominent villous
projections circumferential hairy hairy projections projections
projections
Spleen involvement Red pulp Red pulp White pulp Red pulp
Marrow involvement Interstitial, diffuse Sinusoidal may be Nodular may be Intrasinusoidal, may be
pattern, (fried egg interstitial intrasinusoidal interstitial or nodular
morphology)
Marrow reticulin fibrosis
Marrow reticulin fibrosis Frequent and marked Absent Absent Absent
Immunophenotype by CD11c+, CD19+, CD20+ CD11c+, CD19+, CD20+, CD11c+, CD19+, CD20+, CD11c+/–, CD103+/–,
flow cytometric analysis bright, CD22+, CD25+, CD22+, CD27+, CD79b+, CD22+, CD27+, CD79b+, CD19+. CD20+, kappa or
CD103+,CD123+, FMC7+, CD103+, FMC7+, kappa FMC7+, kappa or lambda lambda+
kappa or lambda (strong) or lambda strong strong Negative for CD25–,
Negative for CD25–, Negative for CD25–, CD123–
CD123– CD123–
Immunophenotype by DBA44+ DBA44+ DBA44+/– DBA44+
immunohistochemistry AnnexinA1+ Annexin A1– Annexin A1– Annexin A1–
Immuno-TRAP+ Immuno-TRAP– Immuno-TRAP– Immuno-TRAP–
Cyclin D1+ Cyclin D – Cyclin D – Cyclin D –
1 1 1
Faint t-Bet+ t-Bet– t-Bet– t-Bet–
V600E BRAF+ V600E BRAF– V600E BRAF– V600E BRAF–
Recurrent mutation V600E BRAF None None None
Somatic hypermutation >85% of cases Mostly >50% of cases Variable
of immunoglobulin
HCL, hairy cell leukemia; HCL-v, variant of hairy cell leukemia; SDRPSBL, splenic diffuse red pulp small B-cell lymphoma; SMZL, splenic marginal
zone lymphoma; t-Bet, T-box transcription factor.
followed by administration of approximately a liter of fluid. Weekly blood or the marrow. MRD is defined as evidence of leukemic cells
19
9,62
blood counts are monitored, and the second and subsequent doses on the marrow biopsy that can be detected using IHC when there is no
are administered if the absolute granulocyte count has not decreased residual morphologic evidence of disease. IHC directed at markers on the
to dangerously low levels. Titrating these doses to be given every 2 leukemic cells may identify residual disease that is either diffusely infil-
to 3 weeks may lessen the degree of myelosuppression related to the trating the marrow or localized. Antibodies directed at CD20, annexin,
agent. After several reduced or delayed doses, the dose and schedule BRAF V600E (e.g., VE1), or DBA.44 will detect disease that is not iden-
62
are returned to the standard dose of 4 mg/m intravenous every 2 weeks tified morphologically. In addition, detailed flow cytometric immuno-
2
19
in an effort to achieve complete remission. The complete remission rate phenotypic analysis of either the blood or the marrow aspirate may be
to pentostatin with this approach approximated 75 percent in a multi- capable of identifying residual leukemia cells (e.g., positive for CD20+,
institutional study. Patients may require 6 months or more of therapy CD11c+, CD103+, CD25+, CD123+ and negative for CD27−). Flow
9
with this agent. When the blood counts and the spleen have returned to cytometry of the marrow may be negatively impacted by the difficulty
normal, then a marrow biopsy should be performed to see if complete in securing an aspirate that is not contaminated by blood. Consequently,
remission has been achieved by morphologic evaluation. This biopsy identification of the extent of MRD using IHC on a marrow biopsy may
will serve as a baseline for evaluation of MRD. If there are no visible be less adversely impacted by sampling error. Eradication of MRD may be
areas of HCL by morphologic criteria, then two additional doses are achieved by adding additional therapy (e.g., administration of rituximab),
administered as consolidation. but the necessity for this additional therapy must be considered. 71
MINIMAL RESIDUAL DISEASE FOLLOWUP CARE
72
Although a complete remission is based upon recovery of blood counts, The advantages of achieving a complete remission have been stressed.
there must be no morphologic evidence of leukemic cells either in the However, the therapy for this disease is immunosuppressive. Extensive
Kaushansky_chapter 93_p1553-1562.indd 1558 9/18/15 3:47 PM