Page 1719 - Williams Hematology ( PDFDrive )
P. 1719
1694 Part XI: Malignant Lymphoid Diseases Chapter 104: Mature T-Cell and Natural Killer Cell Lymphomas 1695
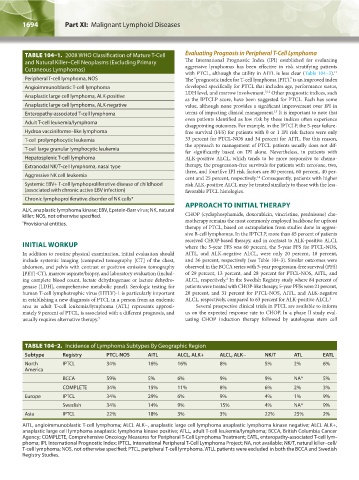
TABLE 104–1. 2008 WHO Classification of Mature T-Cell Evaluating Prognosis in Peripheral T-Cell Lymphoma
and Natural Killer–Cell Neoplasms (Excluding Primary The International Prognostic Index (IPI) established for evaluating
aggressive lymphomas has been effective in risk stratifying patients
Cutaneous Lymphomas)
with PTCL, although the utility in AITL is less clear (Table 104–3).
11
Peripheral T-cell lymphoma, NOS The “prognostic index for T-cell lymphoma (PIT),” is an improved index
Angioimmunoblastic T-cell lymphoma developed specifically for PTCL that includes age, performance status,
LDH level, and marrow involvement. Other prognostic indices, such
7,12
Anaplastic large cell lymphoma, ALK-positive
as the IPTCLP score, have been suggested for PTCL. Each has some
Anaplastic large cell lymphoma, ALK-negative value, although none provides a significant improvement over IPI in
13
Enteropathy-associated T-cell lymphoma terms of impacting clinical management. It is important to note that
even patients identified as low risk by these indices often experience
Adult T-cell leukemia/lymphoma
disappointing outcomes. For example, in the IPTCLP, the 5-year failure-
Hydroa vacciniforme–like lymphoma free survival (FFS) for patients with 0 or 1 IPI risk factors were only
T-cell prolymphocytic leukemia 33 percent for PTCL-NOS and 34 percent for AITL. For this reason,
the approach to management of PTCL patients usually does not dif-
T-cell large granular lymphocytic leukemia
fer significantly based on IPI alone. Nevertheless, in patients with
Hepatosplenic T-cell lymphoma ALK-positive ALCL, which tends to be more responsive to chemo-
Extranodal NK/T-cell lymphoma, nasal type therapy, the progression-free survivals for patients with zero/one, two,
three, and four/five IPI risk factors are 80 percent, 60 percent, 40 per-
Aggressive NK cell leukemia cent and 25 percent, respectively. Consequently, patients with higher
14
Systemic EBV+ T-cell lymphoproliferative disease of childhood risk ALK-positive ALCL may be treated similarly to those with the less-
(associated with chronic active EBV infection) favorable PTCL histologies.
Chronic lymphoproliferative disorder of NK cells*
APPROACH TO INITIAL THERAPY
ALK, anaplastic lymphoma kinase; EBV, Epstein-Barr virus; NK, natural
killer; NOS, not otherwise specified. CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) che-
* Provisional entities. motherapy remains the most commonly employed backbone for upfront
therapy of PTCL, based on extrapolation from studies done in aggres-
sive B-cell lymphomas. In the IPTCLP, more than 85 percent of patients
received CHOP-based therapy, and in contrast to ALK-positive ALCL
INITIAL WORKUP where the 5-year FFS was 60 percent, the 5-year FFS for PTCL-NOS,
In addition to routine physical examination, initial evaluation should AITL, and ALK-negative ALCL, were only 20 percent, 18 percent,
include systemic imaging (computed tomography [CT] of the chest, and 36 percent, respectively (see Table 104-3). Similar outcomes were
abdomen, and pelvis with contrast or positron emission tomography observed in the BCCA series with 5-year progression-free survival (PFS)
[PET]-CT), marrow aspirate/biopsy, and laboratory evaluation (includ- of 29 percent, 13 percent, and 28 percent for PTCL-NOS, AITL, and
ing complete blood count, lactate dehydrogenase or lactate dehydro- ALCL, respectively. In the Swedish Registry study where 84 percent of
2
genase [LDH], comprehensive metabolic panel). Serologic testing for patients were treated with CHOP-like therapy, 5-year PFSs were 21 percent,
human T-cell lymphotrophic virus (HTLV)-1 is particularly important 20 percent, and 31 percent for PTCL-NOS, AITL, and ALK-negative
in establishing a new diagnosis of PTCL in a person from an endemic ALCL, respectively, compared to 63 percent for ALK-positive ALCL. 4
area as adult T-cell leukemia/lymphoma (ATL) represents approxi- Several prospective clinical trials in PTCL are available to inform
mately 9 percent of PTCL, is associated with a different prognosis, and us on the expected response rate to CHOP. In a phase II study eval-
usually requires alternative therapy. 3 uating CHOP induction therapy followed by autologous stem cell
TABLE 104–2. Incidence of Lymphoma Subtypes By Geographic Region
Subtype Registry PTCL-NOS AITL ALCL, ALK+ ALCL, ALK− NK/T ATL EATL
North IPTCL 34% 16% 16% 8% 5% 2% 6%
America
BCCA 59% 5% 6% 9% 9% NA* 5%
COMPLETE 34% 15% 11% 8% 6% 2% 3%
Europe IPTCL 34% 29% 6% 9% 4% 1% 9%
Swedish 34% 14% 9% 15% 4% NA* 9%
Asia IPTCL 22% 18% 3% 3% 22% 25% 2%
AITL, angioimmunoblastic T-cell lymphoma; ALCL ALK−, anaplastic large cell lymphoma anaplastic lymphoma kinase negative; ALCL ALK+,
anaplastic large cell lymphoma anaplastic lymphoma kinase positive; ATLL, adult T-cell leukemia/lymphoma; BCCA, British Columbia Cancer
Agency; COMPLETE, Comprehensive Oncology Measures for Peripheral T-Cell Lymphoma Treatment; EATL, enteropathy-associated T-cell lym-
phoma; IPI, International Prognostic Index; IPTCL, International Peripheral T-Cell Lymphoma Project; NA, not available; NK/T, natural killer–cell/
T-cell lymphoma; NOS, not otherwise specified; PTCL, peripheral T-cell lymphoma. ATLL patients were excluded in both the BCCA and Swedish
*
Registry Studies.
Kaushansky_chapter 104_p1693-1706.indd 1694 9/21/15 12:47 PM