Page 1720 - Williams Hematology ( PDFDrive )
P. 1720
1694 Part XI: Malignant Lymphoid Diseases Chapter 104: Mature T-Cell and Natural Killer Cell Lymphomas 1695
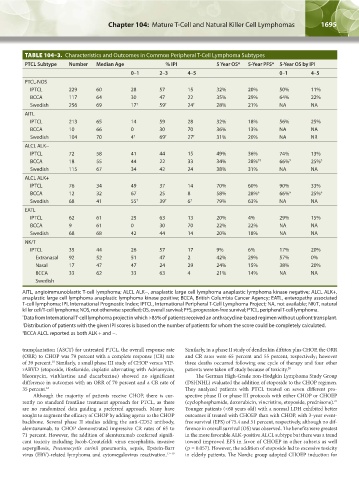
TABLE 104–3. Characteristics and Outcomes in Common Peripheral T-Cell Lymphoma Subtypes
PTCL Subtype Number Median Age % IPI 5 Year OS* 5-Year PFS* 5-Year OS by IPI
0–1 2–3 4–5 0–1 4–5
PTCL-NOS
IPTCL 229 60 28 57 15 32% 20% 50% 11%
BCCA 117 64 30 47 22 35% 29% 64% 22%
Swedish 256 69 17 † 59 † 24 † 28% 21% NA NA
AITL
IPTCL 213 65 14 59 28 32% 18% 56% 25%
BCCA 10 66 0 30 70 36% 13% NA NA
Swedish 104 70 4 † 69 † 27 † 31% 20% NA NR
ALCL ALK−
IPTCL 72 58 41 44 15 49% 36% 74% 13%
BCCA 18 55 44 22 33 34% 28% ‡‡ 66% ‡ 25% ‡
Swedish 115 67 34 42 24 38% 31% NA NA
ALCL ALK+
IPTCL 76 34 49 37 14 70% 60% 90% 33%
BCCA 12 32 67 25 8 58% 28% ‡ 66% ‡ 25% ‡
Swedish 68 41 55 † 39 † 6 † 79% 63% NA NA
EATL
IPTCL 62 61 25 63 13 20% 4% 29% 15%
BCCA 9 61 0 30 70 22% 22% NA NA
Swedish 68 68 42 44 14 20% 18% NA NA
NK/T
IPTCL 35 44 26 57 17 9% 6% 17% 20%
Extranasal 92 52 51 47 2 42% 29% 57% 0%
Nasal 17 47 47 24 29 24% 15% 38% 20%
BCCA 33 62 33 63 4 21% 14% NA NA
Swedish
AITL, angioimmunoblastic T-cell lymphoma; ALCL ALK−, anaplastic large cell lymphoma anaplastic lymphoma kinase negative; ALCL ALK+,
anaplastic large cell lymphoma anaplastic lymphoma kinase positive; BCCA, British Columbia Cancer Agency; EATL, enteropathy associated
T-cell lymphoma; IPI, International Prognostic Index; IPTCL, International Peripheral T-Cell Lymphoma Project; NA, not available; NK/T, natural
killer cell/T-cell lymphoma; NOS, not otherwise specified; OS, overall survival; PFS, progression-free survival; PTCL, peripheral T-cell lymphoma.
* Data from International T-cell lymphoma project in which >85% of patients received an anthracycline-based regimen without upfront transplant.
† Distribution of patients with the given IPI scores is based on the number of patients for whom the score could be completely calculated.
‡ BCCA ALCL reported as both ALK + and −.
transplantation (ASCT) for untreated PTCL, the overall response rate Similarly, in a phase II study of denileukin diftitox plus CHOP, the ORR
(ORR) to CHOP was 79 percent with a complete response (CR) rate and CR rates were 65 percent and 55 percent, respectively; however
of 39 percent. Similarly, a small phase III study of CHOP versus VIP- three deaths occurred following one cycle of therapy and four other
15
rABVD (etoposide, ifosfamide, cisplatin alternating with Adriamycin, patients were taken off study because of toxicity. 20
bleomycin, vinblastine and dacarbazine) showed no significant The German High-Grade non-Hodgkin Lymphoma Study Group
difference in outcomes with an ORR of 70 percent and a CR rate of (DSHNHL) evaluated the addition of etoposide to the CHOP regimen.
35 percent. 16 They analyzed patients with PTCL treated on seven different pro-
Although the majority of patients receive CHOP, there is cur- spective phase II or phase III protocols with either CHOP or CHOEP
rently no standard frontline treatment approach for PTCL, as there (cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone).
21
are no randomized data guiding a preferred approach. Many have Younger patients (<60 years old) with a normal LDH exhibited better
sought to augment the efficacy of CHOP by adding agents to the CHOP outcomes if treated with CHOEP than with CHOP, with 3-year event-
backbone. Several phase II studies adding the anti-CD52 antibody, free survival (EFS) of 75.4 and 51 percent, respectively, although no dif-
alemtuzumab, to CHOP demonstrated impressive CR rates of 65 to ference in overall survival (OS) was observed. The benefits were greatest
71 percent. However, the addition of alemtuzumab conferred signifi- in the more favorable ALK-positive ALCL subtype but there was a trend
cant toxicity including Jacob-Creutzfeldt virus encephalitis, invasive toward improved EFS in favor of CHOEP in other subsets as well
aspergillosis, Pneumocystis carinii pneumonia, sepsis, Epstein-Barr (p = 0.057). However, the addition of etoposide led to excessive toxicity
virus (EBV)-related lymphoma and cytomegalovirus reactivation. 17–19 in elderly patients. The Nordic group adopted CHOEP induction for
Kaushansky_chapter 104_p1693-1706.indd 1695 9/21/15 12:47 PM