Page 1835 - Williams Hematology ( PDFDrive )
P. 1835
1810 Part XI: Malignant Lymphoid Diseases Chapter 110: Heavy-Chain Disease 1811
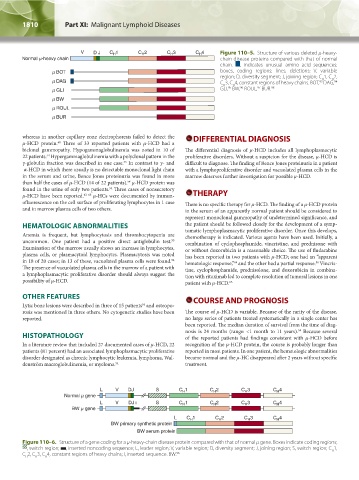
Figure 110–5. Structure of various deleted μ-heavy-
- chain disease proteins compared with that of normal
chain. , indicates unusual amino acid sequences;
boxes, coding regions; lines, deletions; V, variable
region; D, diversity segment; J, joining region; C 1, C 2,
H
H
94
C 3, C 4, constant regions of heavy chains. BOT, DAG,
93
H
H
97
96
95
GLI, BW, ROUL, BUR. 98
whereas in another capillary zone electrophoresis failed to detect the DIFFERENTIAL DIAGNOSIS
μ-HCD protein. Three of 33 reported patients with μ-HCD had a
60
biclonal gammopathy. Hypogammaglobulinemia was noted in 10 of The differential diagnosis of μ-HCD includes all lymphoplasmacytic
22 patients. Hypergammaglobulinemia with a polyclonal pattern in the proliferative disorders. Without a suspicion for the disease, μ-HCD is
54
γ-globulin fraction was described in one case. In contrast to γ- and difficult to diagnose. The finding of Bence Jones proteinuria in a patient
55
α-HCD in which there usually is no detectable monoclonal light chain with a lymphoproliferative disorder and vacuolated plasma cells in the
in the serum and urine, Bence Jones proteinuria was found in more marrow deserves further investigation for possible μ-HCD.
than half the cases of μ-HCD (14 of 22 patients). μ-HCD protein was
54
found in the urine of only two patients. Three cases of nonsecretory
54
μ-HCD have been reported. 61–63 μ-HCs were documented by immun- THERAPY
ofluorescence on the cell surface of proliferating lymphocytes in 1 case There is no specific therapy for μ-HCD. The finding of a μ-HCD protein
and in marrow plasma cells of two others. in the serum of an apparently normal patient should be considered to
represent monoclonal gammopathy of undetermined significance, and
HEMATOLOGIC ABNORMALITIES the patient should be followed closely for the development of a symp-
Anemia is frequent, but lymphocytosis and thrombocytopenia are tomatic lymphoplasmacytic proliferative disorder. Once this develops,
chemotherapy is indicated. Various agents have been used. Initially, a
uncommon. One patient had a positive direct antiglobulin test. combination of cyclophosphamide, vincristine, and prednisone with
55
Examination of the marrow usually shows an increase in lymphocytes, or without doxorubicin is a reasonable choice. The use of fludarabine
plasma cells, or plasmacytoid lymphocytes. Plasmacytosis was noted has been reported in two patients with μ-HCD; one had an “apparent
in 18 of 20 cases; in 13 of these, vacuolated plasma cells were found. hematologic response,” and the other had a partial response. Vincris-
54
56
64
The presence of vacuolated plasma cells in the marrow of a patient with tine, cyclophosphamide, prednisolone, and doxorubicin in combina-
a lymphoplasmacytic proliferative disorder should always suggest the tion with rituximab led to complete resolution of tumoral lesions in one
possibility of μ-HCD. patient with μ-HCD. 65
OTHER FEATURES COURSE AND PROGNOSIS
Lytic bone lesions were described in three of 15 patients and osteopo-
54
rosis was mentioned in three others. No cytogenetic studies have been The course of μ-HCD is variable. Because of the rarity of the disease,
reported. no large series of patients treated systematically in a single center has
been reported. The median duration of survival from the time of diag-
nosis is 24 months (range: <1 month to 11 years). Because several
54
HISTOPATHOLOGY of the reported patients had findings consistent with μ-HCD before
In a literature review that included 27 documented cases of μ-HCD, 22 recognition of the μ-HCD protein, the course is probably longer than
patients (81 percent) had an associated lymphoplasmacytic proliferative reported in most patients. In one patient, the hematologic abnormalities
disorder designated as chronic lymphocytic leukemia, lymphoma, Wal- became normal and the μ-HC disappeared after 2 years without specific
denström macroglobulinemia, or myeloma. 54 treatment.
Figure 110–6. Structure of a gene coding for a μ-heavy-chain disease protein compared with that of normal μ gene. Boxes indicate coding regions;
, switch region; , inserted noncoding sequence; L, leader region; V, variable region; D, diversity segment; J, joining region; S, switch region; C 1,
H
C 2, C 3, C 4, constant regions of heavy chains; I, inserted sequence. BW. 96
H H H
Kaushansky_chapter 110_p1803-1812.indd 1810 9/18/15 10:00 AM