Page 1892 - Williams Hematology ( PDFDrive )
P. 1892
1866 Part XII: Hemostasis and Thrombosis Chapter 112: Platelet Morphology, Biochemistry, and Function 1867
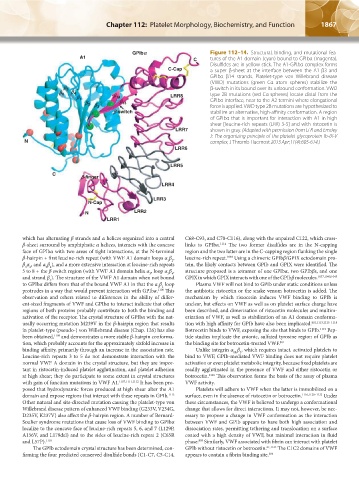
GPlb` Figure 112–14. Structural, binding, and mutational fea-
A1 C tures of the A1 domain (cyan) bound to GPIbα (magenta).
Disulfides are in yellow stick. The A1-GPIbα complex forms
C-Cap a super β-sheet at the interface between the A1 β3 and
GPIbα β14 strands. Platelet-type von Willebrand disease
(VWD) mutations (green Cα atom spheres) stabilize the
a 5 β-switch in its bound over its unbound conformation. VWD
a 6 a 4 type 2B mutations (red Cα spheres) locate distal from the
a 1 a 14 LRR8 GPIbα interface, near to the A2 termini where elongational
a 2 a 3 a 13 force is applied. VWD type 2B mutations are hypothesized to
a-switch stabilize an alternative, high-affinity conformation. A region
of GPIbα that is important for interaction with A1 in high
shear [leucine-rich repeats (LRR) 3-5] and with ristocetin is
LRR7 shown in gray. (Adapted with permission from Li R and Emsley
J: The organizing principle of the platelet glycoprotein Ib-IX-V
N complex, J Thromb Haemost 2013 Apr;11(4):605-614.)
LRR6
LRR5
C
a-finger
LRR4
LRR3
N-Cap
N LRR2
LRR1
which has alternating β strands and α helices organized into a central C68-C93, and C70-C116), along with the unpaired C122, which cross-
β-sheet surround by amphiphatic α helices, interacts with the concave links to GPIbα. 1114 The two former disulfides are in the N-capping
face of GPIbα with two areas of tight interactions, at the N-terminal region and the two latter are in the C-capping region flanking the single
β-hairpin + first leucine-rich repeat (with VWF A1 domain loops α β , leucine-rich repeat. 1040 Using a chimeric GPIbβ/GPIX ectodomain pro-
1 2
β α , and α β ), and a more extensive interaction at leucine-rich repeats tein, the likely contacts between GPIb and GPIX were identified. The
3 4
3 2
5 to 8 + the β switch region (with VWF A1 domain helix α , loop α β , structure proposed is a tetramer of one GPIbα, two GPIbβs, and one
3 4
3
and strand β ). The structure of the VWF A1 domain when not bound GPIX in which GPIX interacts with one of the GPIbβ molecules. 1037,1040,1043
3
to GPIbα differs from that of the bound VWF A1 in that the α β loop Plasma VWF will not bind to GPIb under static conditions unless
1 2
protrudes in a way that would prevent interaction with GPIbα. 1108 This the antibiotic ristocetin or the snake venom botrocetin is added. The
observation and others related to differences in the ability of differ- mechanism by which ristocetin induces VWF binding to GPIb is
ent-sized fragments of VWF and GPIbα to interact indicate that other unclear, but effects on VWF as well as on platelet surface charge have
regions of both proteins probably contribute to both the binding and been described, and dimerization of ristocetin molecules and multim-
activation of the receptor. The crystal structure of GPIbα with the nat- erization of VWF, as well as stabilization of an A1 domain conforma-
urally occurring mutation M239V in the β-hairpin region that results tion with high affinity for GPIb have also been implicated. 801,1113,1115–1118
in platelet-type (pseudo-) von Willebrand disease (Chap. 126) has also Botrocetin binds to VWF, exposing the site that binds to GPIb. 1119 Pep-
been obtained, 1109 and demonstrates a more stable β-hairpin conforma- tide studies implicate the anionic, sulfated tyrosine region of GPIb as
tion, which probably accounts for the approximately sixfold increase in the binding site for botrocetin-treated VWF. 801
binding affinity, primarily through an increase in the association rate. Unlike integrin α β , which requires intact, activated platelets to
IIb 3
Leucine-rich repeats 3 to 5 do not demonstrate interaction with the bind to VWF, GPIb-mediated VWF binding does not require platelet
normal VWF A domain in the crystal structure, but they are impor- activation or even platelet metabolic integrity, because fixed platelets are
tant in ristocetin-induced platelet agglutination, and platelet adhesion readily agglutinated in the presence of VWF and either ristocetin or
at high shear; they do participate to some extent in crystal structures botrocetin. 1116 This observation forms the basis of the assay of plasma
with gain of function mutations in VWF A1. 1107,1111,1112 It has been pro- VWF activity.
posed that hydrodynamic forces produced at high shear alter the A1 Platelets will adhere to VWF when the latter is immobilized on a
domain and expose regions that interact with these repeats in GPIb. 1113 surface, even in the absence of ristocetin or botrocetin. 1116,1120–1122 Under
Other natural and site-directed mutation causing the platelet-type von these circumstances, the VWF is believed to undergo a conformational
Willebrand disease pattern of enhanced VWF binding (G233V, V234G, change that allows for direct interactions. It may not, however, be nec-
D235V, K237V) also affect the β-hairpin region. A number of Bernard- essary to propose a change in VWF conformation as the interaction
Soulier syndrome mutations that cause loss of VWF binding to GPIbα between VWF and GPIb appears to have both high association and
localize to the concave face of leucine-rich repeats 5, 6, and 7 (L129P, dissociation rates, permitting tethering and translocation on a surface
A156V, and L179del) and to the sides of leucine-rich repeat 2 (C65R coated with a high density of VWF, but minimal interaction in fluid
and L57P). 1110 phase. Similarly, VWF associated with fibrin can interact with platelet
809
The GPIb ectodomain crystal structure has been determined, con- GPIb without ristocetin or botrocetin. 61,1123 The C1C2 domains of VWF
firming the four predicted conserved disulfide bonds (C1-C7, C5-C14, appears to contain a fibrin binding site. 304
Kaushansky_chapter 112_p1829-1914.indd 1867 17/09/15 3:29 pm