Page 1891 - Williams Hematology ( PDFDrive )
P. 1891
1866 Part XII: Hemostasis and Thrombosis Chapter 112: Platelet Morphology, Biochemistry, and Function 1867
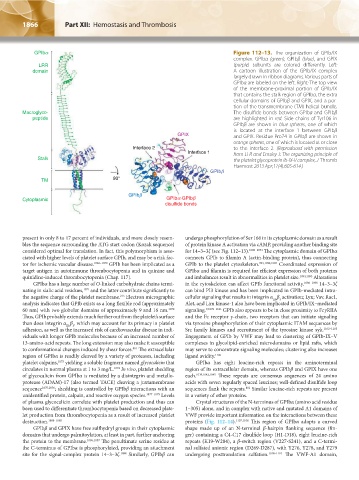
GPIba Figure 112–13. The organization of GPIb/IX
complex. GPIbα (green), GPIbβ (blue), and GPIX
LRR (purple) subunits are colored differently. Left:
domain A cartoon illustration of the GPIb/IX complex
largely drawn in ribbon diagrams. Various parts of
GPIbα are labeled on the left. Right: The top view
of the membrane-proximal portion of GPIb/IX
that contains the stalk region of GPIbα, the extra
cellular domains of GPIbβ and GPIX, and a por-
tion of the transmembrane (TM) helical bundle.
Macroglyco- The disulfide bonds between GPIbα and GPIbβ
peptide are highlighted in red. Side chains of Tyr106 in
GPIbβ are shown in blue spheres, one of which
is located at the interface 1 between GPIbβ
GPIX and GPIX. Residue Pro74 in GPIbβ are shown in
orange spheres, one of which is located at or close
Interface 2 to the interface 2. (Reproduced with permission
Interface 1 from Li R and Emsley J: The organizing principle of
Stalk the platelet glycoprotein Ib-IX-V complex, J Thromb
Haemost 2013 Apr;11(4):605-614.)
GPIbb
TM 90°
GPIbb
Cytoplasmic GPIba-GPIbb
disulfide bonds
present in only 8 to 17 percent of individuals, and more closely resem- undergo phosphorylation of Ser 166 in its cytoplasmic domain as a result
bles the sequence surrounding the ATG start codon (Kozak sequence) of protein kinase A activation via cAMP, providing another binding site
considered optimal for translation. In fact, this polymorphism is asso- for 14–3–3ζ (see Fig. 112–13). 1089–1091 The cytoplasmic domain of GPIbα
ciated with higher levels of platelet surface GPIb, and may be a risk fac- connects GPIb to filamin A (actin-binding protein), thus connecting
tor for ischemic vascular disease. 1062–1070 GPIb has been implicated as a GPIb to the platelet cytoskeleton. 993,1092,1093 Coordinated expression of
target antigen in autoimmune thrombocytopenia and in quinine and GPIbα and filamin is required for efficient expression of both proteins
quinidine-induced thrombocytopenia (Chap. 117). and imbalances result in abnormalities in platelet size. 1094,1095 Alterations
GPIbα has a large number of O-linked carbohydrate chains termi- in the cytoskeleton can affect GPIb functional activity. 1096–1098 14–3–3ζ
nating in sialic acid residues, 1071 and the latter contribute significantly to can bind PI3 kinase and has been implicated in GPIb-mediated intra-
the negative charge of the platelet membrane. Electron micrographic cellular signaling that results in integrin α β activation; Lyn; Vav, Rac1,
215
IIb 3
analysis indicates that GPIb exists as a long flexible rod (approximately Alet, and Lim kinase-1 also have been implicated in GPIb/IX–mediated
60 nm) with two globular domains of approximately 9 and 16 nm. 1072 signaling. 9,1099–1101 GPIb also appears to be in close proximity to FcγRIIA
Thus, GPIb probably extends much further out from the platelet’s surface and the Fc receptor γ-chain, two receptors that can initiate signaling
than does integrin α β , which may account for its primacy in platelet via tyrosine phosphorylation of their cytoplasmic ITAM sequences by
IIb 3
adhesion, as well as the increased risk of cardiovascular disease in indi- Src family kinases and recruitment of the tyrosine kinase syk. 1102–1105
viduals with longer GPIb molecules because of an increased number of Engagement of GPIb by VWF may lead to clustering of GPIb-IX–V
13-amino-acid repeats. The long extension may also make it susceptible complexes in glycolipid-enriched microdomains or lipid rafts, which
801
to conformational changes induced by shear forces. The extracellular may serve to concentrate signaling molecules; clustering also increases
region of GPIbα is readily cleaved by a variety of proteases, including ligand avidity. 1106
platelet calpains, 1073 yielding a soluble fragment named glycocalicin that GPIbα has eight leucine-rich repeats in the aminoterminal
circulates in normal plasma at 1 to 3 mg/L. 1074 In vivo, platelet shedding region of its extracellular domain, whereas GPIbβ and GPIX have one
of glycocalicin from GPIbα is mediated by a disintegrin and metallo- each. 1039,1042,1045 These repeats are consensus sequences of 24 amino
protease (ADAM)-17 (also termed TACE) cleaving a juxtamembrane acids with seven regularly spaced leucines; well-defined disulfide loop
801
sequence 1075,1076 ; shedding is controlled by GPIbβ interactions with an sequences flank the repeats. Similar leucine-rich repeats are present
unidentified protein, calpain, and reactive oxygen species. 1077–1079 Levels in a variety of other proteins.
of plasma glycocalicin correlate with platelet production and thus can Crystal structures of the N-terminus of GPIbα (amino acid residue
been used to differentiate thrombocytopenia based on decreased plate- 1–305) alone, and in complex with native and mutated A1 domains of
let production from thrombocytopenia as a result of increased platelet VWF provide important information on the interactions between these
destruction. 1080–1085 proteins (Fig. 112–14). 1107,1108 This region of GPIbα adopts a curved
GPIbβ and GPIX have free sulfhydryl groups in their cytoplasmic shape made up of an N-terminal β-hairpin flanking sequence (fin-
domains that undergo palmitoylation, at least in part, further anchoring ger) containing a C4-C17 disulfide loop (H1-D18), eight leucine-rich
the protein to the membrane. 1086,1087 The penultimate serine residue at repeats (K19-W204), a β-switch region (V227-S241), and a C-termi-
the C-terminus of GPIbα is phosphorylated, providing an attachment nal sulfated anionic region (D269-D287), with Y276, Y278, and Y279
site for the signal-complex protein 14–3–3ζ. 1088 Similarly, GPIbβ can undergoing posttranslation sulfation. 1108–1110 The VWF-A1 domain,
Kaushansky_chapter 112_p1829-1914.indd 1866 17/09/15 3:29 pm