Page 1888 - Williams Hematology ( PDFDrive )
P. 1888
1862 Part XII: Hemostasis and Thrombosis Chapter 112: Platelet Morphology, Biochemistry, and Function 1863
membrane clasp and subsequent dissociation of the transmembrane helices. fibrinogen γ-chain C-terminal peptide better, with the peptide’s Asp and
This potentially may facilitate the interaction of the cytoplasmic C-terminal Val carboxyls interacting with the MIDAS and ADMIDAS
domains with cytoskeletal elements and signaling molecules. cations, respectively. It also explains why integrin α β can bind pep-
826
IIb 3
923
The integrin β tail also contains two NXXY motifs and Y747 and tides containing the longer Lys residue (KGD peptides). Crystal struc-
3
Y759 within one of these motifs are phosphorylated upon platelet aggre- tures are also available for the integrin α β receptor with the drugs
IIb 3
gation, thus producing docking sites for signaling molecules. Studies eptifibatide and tirofiban, which are effective antithrombotic agents
235
in mice and in recombinant systems demonstrate a role for the sites in because of their ability to block ligand binding to integrin α β , and
IIb 3
clot retraction and platelet aggregate stability. 291,902 demonstrate specificity for integrin α β compared to integrin α β .
827
V 3
IIb 3
A number of proteins have been shown to bind to the cytoplasmic The basis of the specificity of these agents involves in part their interac-
domains of integrin α and/or β , either directly or through interac- tion with the integrin α -specific exosite and the greater length between
IIb
3
IIb
tions with other proteins, including signaling molecules (Src, Shc, FAK, their positive and negative charges. The third integrin α β antagonist
827
IIb 3
paxillin, and ILK, all of which bind to integrin β ), cytoskeletal proteins drug, abciximab, is a chimeric murine monoclonal antibody Fab frag-
3
(kindlin-3, skelemin, α-actin, and myosin, which bind to integrin β , ment. Its epitope has been localized to a region on integrin β very close
3
3
and filamin and talin, which bind to integrins α and/or β ), and other to the MIDAS, suggesting that it works by steric interference with ligand
IIb
3
proteins (β -endonexin and CD98, which bind to integrin β , and CIB binding, disruption of the binding pocket, or both mechanisms.
3
3
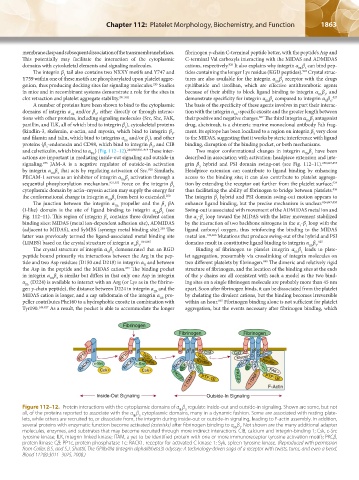
and calreticulin, which bind to α ) (Fig. 112–12). 244,866,903–919 These inter- Two major conformational changes in integrin α β have been
IIb 3
IIb
actions are important in mediating inside-out signaling and outside-in described in association with activation: headpiece extension and inte-
235
signaling. JAM-A is a negative regulator of outside-in activation grin β hybrid and PSI domain swing-out (see Fig. 112–11). 250,827,853
3
by integrin α β that acts by regulating activation of Src. Similarly, Headpiece extension can contribute to ligand binding by enhancing
920
IIb 3
PECAM-1 serves as an inhibitor of integrin α β activation through a access to the binding site; it can also contribute to platelet aggrega-
IIb 3
sequential phosphorylation mechanism. 921,922 Force on the integrin β tion by extending the receptor out further from the platelet surface,
924
3
846
cytoplasmic domain by actin–myosin action may supply the energy for thus facilitating the ability of fibrinogen to bridge between platelets.
the conformational change in integrin α β from bent to extended. 250 The integrin β hybrid and PSI domain swing-out motion appears to
3
IIb 3
The junction between the integrin α propeller and the β βA enhance ligand binding, but the precise mechanism is unclear. 826,847,850
IIb
3
(I-like) domain is the site of ligand binding to integrin α β (see Swing-out is associated with movement of the ADMIDAS metal ion and
IIb 3
Fig. 112–11). This region of integrin β contains three divalent cation the α -β loop toward the MIDAS with the latter movement stabilized
1
1
3
binding sites: MIDAS (metal ion-dependent adhesion site), ADMIDAS by the interaction of two backbone nitrogens in the α -β loop with the
1
1
250
(adjacent to MIDAS), and SyMBS (synergy metal binding site). The ligand carboxyl oxygen, thus reinforcing the binding to the MIDAS
latter was previously termed the ligand-associated metal binding site metal ion. 149,826 Mutations that produce swing-out of the hybrid and PSI
(LIMBS) based on the crystal structure of integrin α β . 844,845 domains result in constitutive ligand binding to integrin α β . 925
IIb 3
V 3
The crystal structure of integrin α β demonstrated that an RGD Binding of fibrinogen to platelet integrin α β leads to plate-
V 3
IIb 3
peptide bound primarily via interactions between the Arg in the pep- let aggregation, presumably via crosslinking of integrin molecules on
tide and two Asp residues (D150 and D218) in integrin α and between two different platelets by fibrinogen. The dimeric and relatively rigid
840
V
845
the Asp in the peptide and the MIDAS cation. The binding pocket structure of fibrinogen, and the location of the binding sites at the ends
in integrin α β is similar but differs in that only one Asp in integrin of the γ chains are all consistent with such a model as the two bind-
IIb 3
α (D224) is available to interact with an Arg (or Lys as in the fibrino- ing sites on a single fibrinogen molecule are probably more than 45 nm
IIb
gen γ-chain peptide), the distance between D224 in integrin α and the apart. Soon after fibrinogen binds, it can be dissociated from the platelet
IIb
MIDAS cation is longer, and a cap subdomain of the integrin α pro- by chelating the divalent cations, but the binding becomes irreversible
IIb
835
peller contributes Phe160 to a hydrophobic exosite in combination with within an hour. Fibrinogen binding alone is not sufficient for platelet
Tyr190. 149,827 As a result, the pocket is able to accommodate the longer aggregation, but the events necessary after fibrinogen binding, which
Inside-Out Signaling Outside-In Signaling
Figure 112–12. Protein interactions with the cytoplasmic domains of α β regulate inside-out and outside-in signaling. Shown are some, but not
IIb 3
all, of the proteins reported to associate with the α β cytoplasmic domains, many in a dynamic fashion. Some are associated with resting plate-
IIb 3
lets, while others are recruited to, or dissociate from, the integrin during inside-out or outside-in signaling, leading to F-actin assembly. In addition,
several proteins with enzymatic function become activated (asterisks) after fibrinogen binding to α β . Not shown are the many additional adapter
IIb 3
molecules, enzymes, and substrates that may become recruited through more indirect interactions. CIB, calcium and integrin-binding 1; Csk, c-Src
tyrosine kinase; ILK, integrin-linked kinase; ITAM, a yet-to-be identified protein with one or more immunoreceptor tyrosine activation motifs; PKCβ,
protein kinase Cβ; PP1c, protein phosphatase 1c; RACK1, receptor for activated C kinase 1; Syk, spleen tyrosine kinase. (Reproduced with permission
from Coller, B.S. and S.J. Shattil, The GPIIb/IIIa (integrin alphaIIbbeta3) odyssey: A technology-driven saga of a receptor with twists, turns, and even a bend.
Blood 112(8):3011–3025, 2008.)
Kaushansky_chapter 112_p1829-1914.indd 1863 17/09/15 3:29 pm