Page 1900 - Williams Hematology ( PDFDrive )
P. 1900
1874 Part XII: Hemostasis and Thrombosis Chapter 112: Platelet Morphology, Biochemistry, and Function 1875
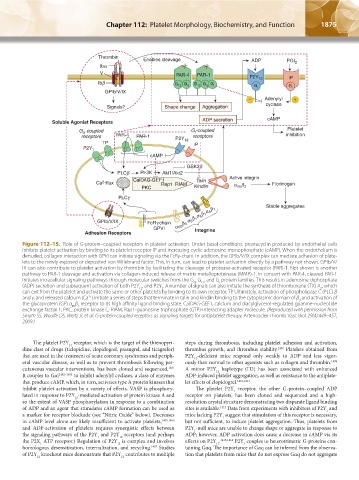
Figure 112–15. Role of G-protein–coupled receptors in platelet activation. Under basal conditions, prostacyclin produced by endothelial cells
inhibits platelet activation by binding to its platelet receptor IP and increasing cyclic adenosine monophosphate (cAMP). When the endothelium is
denuded, collagen interaction with GPVI can initiate signaling via the FcRγ-chain. In addition, the GPIb/V/IX complex can mediate adhesion of plate-
lets to the newly exposed or deposited von Willebrand factor. This, in turn, can lead to platelet activation directly by a pathway not shown. GPIb/V/
IX can also contribute to platelet activation by thrombin by facilitating the cleavage of protease-activated receptor (PAR)-1. Not shown is another
pathway to PAR-1 cleavage and activation via collagen-induced release of matrix metalloproteinase (MMP)-1. In concert with PAR-4, cleaved PAR-1
initiates intracellular signaling pathways through molecular switches from the G , G , and G protein families. This results in adenosine diphosphate
q
12
i
(ADP) secretion and subsequent activation of both P2Y , and P2Y . A number of signals can also initiate the synthesis of thromboxane (TX) A , which
12
2
1
can exit from the platelet and activate the same or other platelets by binding to its own receptor, TP. Ultimately, activation of phospholipase C (PLC) β
and γ, and released calcium (Ca ) initiate a series of steps that terminate in talin and kindlin binding to the cytoplasmic domain of β and activation of
2+
3
the glycoprotein (GP) α β receptor to its high affinity ligand binding state. CalDAG-GEF1, calcium and diacylglycerol-regulated guanine-nucleotide
IIb 3
exchange factor 1; PKC, protein kinase C; RIAM, Rap1-guanosine triphosphate (GTP)-interacting adapter molecule. (Reproduced with permission from
Smyth SS, Woulfe DS, Weitz JI, et al: G-protein-coupled receptors as signaling targets for antiplatelet therapy. Arterioscler Thromb Vasc Biol 29(4):449–457,
2009.)
The platelet P2Y receptor, which is the target of the thienopyri- steps during thrombosis, including platelet adhesion and activation,
12
dine class of drugs (ticlopidine, clopidogrel, prasugrel, and ticagrelor) thrombus growth, and thrombus stability. 1408 Platelets obtained from
that are used in the treatment of acute coronary syndromes and periph- P2Y -deficient mice respond only weakly to ADP and less vigor-
12
eral vascular disease, as well as to prevent thrombosis following per- ously than normal to other agonists such as collagen and thrombin. 1409
cutaneous vascular interventions, has been cloned and sequenced. 1401 A minor P2Y haplotype (H2) has been associated with enhanced
12
It couples to Gαi 1402–1404 to inhibit adenylyl cyclases, a class of enzymes ADP-induced platelet aggregation, as well as resistance to the antiplate-
that produce cAMP, which, in turn, activates type A protein kinases that let effects of clopidogrel. 1410,1411
inhibit platelet activation by a variety of effects. VASP is phosphory- The platelet P2Y receptor, the other G-protein–coupled ADP
1
lated in response to P2Y -mediated activation of protein kinase A and receptor on platelets, has been cloned and sequenced and a high-
12
so the extent of VASP phosphorylation in response to a combination resolution crystal structure demonstrating two disparate ligand binding
of ADP and an agent that stimulates cAMP formation can be used as sites is available. 1412 Data from experiments with inhibitors of P2Y and
1
a marker for receptor blockade (see “Nitric Oxide” below). Decreases mice lacking P2Y suggest that stimulation of this receptor is necessary,
1
in cAMP level alone are likely insufficient to activate platelets, 1405,1406 but not sufficient, to induce platelet aggregation. Thus, platelets from
and ADP-activation of platelets requires synergistic effects between P2Y -null mice are unable to change shape or aggregate in response to
1
the signaling pathways of the P2Y and P2Y receptors (and perhaps ADP; however, ADP activation does cause a decrease in cAMP via its
1
12
the P2X ATP receptor.) Regulation of P2Y is complex and involves effects on P2Y . 1413,1414 P2Y couples to heterotrimeric G-proteins con-
1
12
1
12
homologous desensitization, internalization, and recycling. 1407 Studies taining Gαq. The importance of Gαq can be inferred from the observa-
of P2Y knockout mice demonstrate that P2Y contributes to multiple tion that platelets from mice that do not express Gαq do not aggregate
12 12
Kaushansky_chapter 112_p1829-1914.indd 1875 17/09/15 3:30 pm