Page 1902 - Williams Hematology ( PDFDrive )
P. 1902
1876 Part XII: Hemostasis and Thrombosis Chapter 112: Platelet Morphology, Biochemistry, and Function 1877
contribute to shape change and aggregation. 1469–1471 It is unclear whether Inactive PAR-1 Active PAR-1
TP directly couples to GαI 1472 or activates this pathway indirectly via N
released ADP. 1467,1470 A significant portion of PGH /TXA -induced
2
2
platelet aggregation is actually mediated by secreted ADP, because ADP S
scavenger systems inhibit aggregation induced by a stable PGH /TXA F L Thrombin
2
2
analogue either partially (30 percent) 1473 or totally. 1472 R L Extracellular
AA can also be converted to LTs and lipoxins by the sequential N N RL L F S
actions of LOX and other enzymes. Platelets from most animal species
lack 5-LOX, but possess 12-LOX. Consequently, AA liberated by cyto-
solic PLA α can be oxygenated by 12-LOX to generate 12-HPETE, an
2
unstable intermediate that is reduced by glutathione peroxidase or other
mechanisms to generate HETE. The generation of 12-HPETE in platelets C C
is slower and more sustained than the generation of TXA. 1474 Platelets Intracellular
from mice deficient in the platelet-type 12-LOX are hypersensitive to
stimulation by ADP, suggesting an inhibitory role for this pathway in Gi
platelet activation by ADP. 1475 12-LOX activity in platelets can be regu-
lated by signaling through the GPVI collagen receptor. 1476 Because they G 12/13 G q
lack 5-LOX, platelets do not generate LTB , nor do they appear to possess
4
LTB receptors. 1477 However, they participate in LT and lipoxin (LX) gen-
4
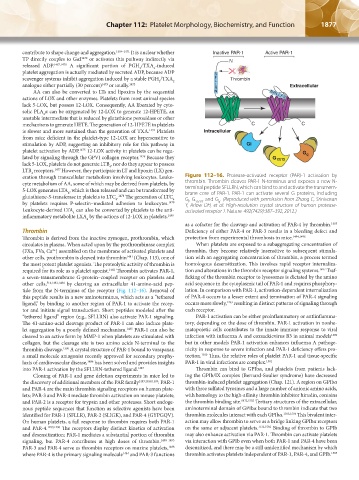
eration through transcellular metabolism involving leukocytes. Leuko- Figure 112–16. Protease-activated receptor (PAR)-1 activation by
cyte metabolism of AA, some of which may be derived from platelets, by thrombin. Thrombin cleaves PAR-1 N-terminus and exposes a new N-
5-LOX generates LTA , which is then released and can be transformed by terminal peptide SFLLRN, which can bind to and activate the transmem-
brane core of PAR-1. PAR-1 can activate several G proteins, including
4
glutathione-S-transferase in platelets to LTC . 1478 The generation of LTC G, G , and G . (Reproduced with permission from Zhang C, Srinivasan
4
4
i
12/13
q
by platelets requires P-selectin–mediated adhesion to leukocytes. 1479 Y, Arlow DH, et al: High-resolution crystal structure of human protease-
Leukocyte-derived LTA can also be converted by platelets to the anti- activated receptor 1. Nature 492(7429):387–392, 2012.)
4
inflammatory metabolite LXA by the actions of 12-LOX in platelets. 1480
4
as a cofactor for the cleavage and activation of PAR-4 by thrombin. 1495
Thrombin Deficiency of either PAR-4 or PAR-3 results in a bleeding defect and
Thrombin is derived from the inactive zymogen, prothrombin, which protection from experimental thrombosis in mice. 1494,1496
circulates in plasma. When acted upon by the prothrombinase complex When platelets are exposed to a subaggregating concentration of
2+
(FXa, FVa, Ca ) assembled on the membrane of activated platelets and thrombin, they become relatively insensitive to subsequent stimula-
other cells, prothrombin is cleaved into thrombin 1481 (Chap. 113), one of tion with an aggregating concentration of thrombin, a process termed
the most potent platelet agonists. The proteolytic activity of thrombin is homologous desensitization. This involves rapid receptor internaliza-
required for its role as a platelet agonist. 1482 Thrombin activates PAR-1, tion and alterations in the thrombin receptor signaling systems. 1497 Traf-
a seven-transmembrane G-protein–coupled receptor on platelets and ficking of the thrombin receptor to lysosomes is dictated by the amino
other cells, 514,1483,1484 by cleaving an extracellular 41-amino-acid pep- acid sequence in the cytoplasmic tail of PAR-1 and requires phosphory-
tide from the N-terminus of the receptor (Fig. 112–16). Removal of lation. In comparison with PAR-1, activation-dependent internalization
this peptide results in a new aminoterminus, which acts as a “tethered of PAR-4 occurs to a lesser extent and termination of PAR-4 signaling
ligand,” by binding to another region of PAR-1 to activate the recep- occurs more slowly, 1493 resulting in distinct patterns of signaling through
tor and initiate signal transduction. Short peptides modeled after the each receptor.
“tethered ligand” region (e.g., SFLLRN) also activate PAR-1 signaling. PAR-1 activation can be either proinflammatory or antiinflamma-
The 41-amino-acid cleavage product of PAR-1 can also induce plate- tory, depending on the dose of thrombin. PAR-1 activation in nonhe-
let aggregation by a poorly defined mechanism. 1485 PAR-1 can also be matopoietic cells contributes to the innate immune response to viral
cleaved to an active form by MMP-1 when platelets are stimulated with infection with influenza A and coxsackievirus B3 in animal models 1499
collagen, but the cleavage site is two amino acids N-terminal to the but in other models PAR-1 activation enhances influenza A pathoge-
thrombin cleavage. 1390 A crystal structure of PAR-1 bound to vorapaxar, nicity in response to severe infection and PAR-1 deficiency offers pro-
a small molecule antagonist recently approved for secondary prophy- tection. 1500 Thus, the relative roles of platelet PAR-1 and tissue-specific
laxis of cardiovascular disease, 1486 has been solved and provides insights PAR-1 in viral infections are complex. 1501
into PAR-1 activation by the SFLLRN-tethered ligand. 1487 Thrombin can bind to GPIbα, and platelets from patients lack-
Cloning of PAR-1 and gene deletion experiments in mice led to ing the GPIb/IX complex (Bernard-Soulier syndrome) have decreased
the discovery of additional members of the PAR family 1483,1488,1489 : PAR-1 thrombin-induced platelet aggregation (Chap. 121). A region on GPIbα
and PAR-4 are the main thrombin signaling receptors on human plate- with three sulfated tyrosines and a large number of anionic amino acids,
lets; PAR-3 and PAR-4 mediate thrombin activation on mouse platelets; with homology to the high-affinity thrombin inhibitor hirudin, contains
and PAR-2 is a receptor for trypsin and other proteases. Short endoge- the thrombin binding site. 1132,1502 Tertiary structures of the extracellular,
nous peptide sequences that function as selective agonists have been aminoterminal domain of GPIbα bound to thrombin indicate that two
identified for PAR-1 (SFLLR), PAR-2 (SLIGK), and PAR-4 (GYPGQV). thrombin molecules interact with each GPIbα. 1503,1504 This bivalent inter-
On human platelets, a full response to thrombin requires both PAR-1 action may allow thrombin to serve as a bridge linking GPIbα receptors
and PAR-4. 1489,1490 The receptors display distinct kinetics of activation on the same or adjacent platelets. 1132,1502 Binding of thrombin to GPIb
and desensitization; PAR-1 mediates a substantial portion of thrombin may also enhance activation via PAR-1. Thrombin can activate platelets
signaling, but PAR-4 contributes at high doses of thrombin. 1490–1493 via interaction with GPIb even when both PAR-1 and PAR-4 have been
PAR-3 and PAR-4 serve as thrombin receptors on murine platelets, 1488 desensitized, and there may be a still unidentified mechanism by which
where PAR-4 is the primary signaling molecule 1494 and PAR-3 functions thrombin activates platelets independent of PAR-1, PAR-4, and GPIb. 1505
Kaushansky_chapter 112_p1829-1914.indd 1877 17/09/15 3:30 pm