Page 1975 - Williams Hematology ( PDFDrive )
P. 1975
1950 Part XII: Hemostasis and Thrombosis Chapter 114: Control of Coagulation Reactions 1951
Inactivation
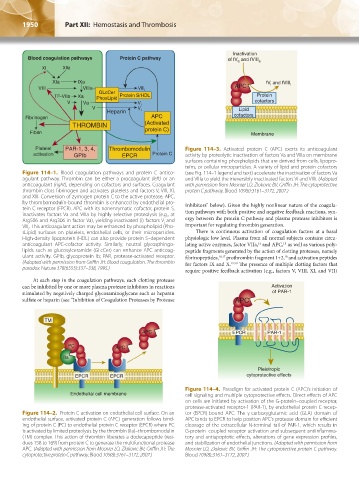
Blood coagulation pathways Protein C pathway of fV and fVIII
a
a
XI XIIa
XIa IXa fV and fVIII i
i
VIII VIIIa VIII i APC
GLcCer
TF-VIIa Xa PhosLipid Protein S/HDL Protein
V Va V i cofactors
V V Lipid
Heparin i
Fibrinogen APC cofactors
THROMBIN (Activated
Fibrin protein C) Membrane
Platelet PAR-1, 3, 4, Thrombomodulin Figure 114–3. Activated protein C (APC) exerts its anticoagulant
activation GPIb EPCR Protein C activity by proteolytic inactivation of factors Va and VIIIa on membrane
surfaces containing phospholipids that are derived from cells, lipopro-
teins, or cellular microparticles. A variety of lipid and protein cofactors
Figure 114–1. Blood coagulation pathways and protein C antico- (see Fig. 114–1 legend and text) accelerate the inactivation of factors Va
agulant pathway. Thrombin can be either a procoagulant (left) or an and VIIIa to yield the irreversibly inactivated factors Vi and VIIIi. (Adapted
anticoagulant (right), depending on cofactors and surfaces. Coagulant with permission from Mosnier LO, Zlokovic BV, Griffin JH: The cytoprotective
thrombin clots fibrinogen and activates platelets and factors V, VIII, XI, protein C pathway. Blood 109(8):3161–3172, 2007.)
and XIII. Conversion of zymogen protein C to the active protease, APC,
by thrombomodulin-bound thrombin is enhanced by endothelial pro- Inhibitors” below). Given the highly nonlinear nature of the coagula-
tein C receptor (EPCR). APC with its nonenzymatic cofactor, protein S, tion pathways with both positive and negative feedback reactions, syn-
inactivates factors Va and VIIIa by highly selective proteolysis (e.g., at
Arg506 and Arg306 in factor Va), yielding inactivated (i) factors V and ergy between the protein C pathway and plasma protease inhibitors is
i
VIII. This anticoagulant action may be enhanced by phospholipid (Pho- important for regulating thrombin generation.
i
sLipid) surfaces on platelets, endothelial cells, or their microparticles. There is continuous activation of coagulation factors at a basal
High-density lipoprotein (HDL) can also provide protein S–dependent physiologic low level. Plasma from all normal subjects contains circu-
anticoagulant APC-cofactor activity. Similarly, neutral glycosphingo- lating active enzymes, factor VIIa, and APC, as well as various poly-
15
14
lipids such as glucosylceramide (GLcCer) can enhance APC anticoag- peptide fragments generated by the action of clotting proteases, namely
ulant activity. GPIb, glycoprotein Ib; PAR, protease-activated receptor. fibrinopeptides, 16,17 prothrombin fragment 1+2, and activation peptides
18
(Adapted with permission from Griffin JH: Blood coagulation. The thrombin for factors IX and X. 19,20 The presence of multiple clotting factors that
paradox. Nature 378(6555):337–338, 1995.)
require positive feedback activation (e.g., factors V, VIII, XI, and VII)
At each step in the coagulation pathways, each clotting protease
can be inhibited by one or more plasma protease inhibitors in reactions Activation
stimulated by negatively charged glycosaminoglycans such as heparan of PAR-1
sulfate or heparin (see “Inhibition of Coagulation Proteases by Protease APC
TM
APC EPCR PAR-1
PC APC
IIa
Pleiotropic
EPCR EPCR cytoprotective effects
Figure 114–4. Paradigm for activated protein C (APC)’s initiation of
Endothelial cell membrane cell signaling and multiple cytoprotective effects. Direct effects of APC
on cells are initiated by activation of the G-protein–coupled receptor,
protease-activated receptor-1 (PAR-1), by endothelial protein C recep-
Figure 114–2. Protein C activation on endothelial cell surface. On an tor (EPCR)-bound APC. The γ-carboxyglutamic acid (GLA) domain of
endothelial surface, activated protein C (APC) generation follows bind- APC binds to EPCR to help position APC’s protease domain for efficient
ing of protein C (PC) to endothelial protein C receptor (EPCR) where PC cleavage of the extracellular N-terminal tail of PAR-1, which results in
is activated by limited proteolysis by the thrombin (IIa)–thrombomodulin G-protein–coupled receptor activation and subsequent antiinflamma-
(TM) complex. This action of thrombin liberates a dodecapeptide (resi- tory and antiapoptotic effects, alterations of gene expression profiles,
dues 158 to 169) from protein C to generate the multifunctional protease and stabilization of endothelial junctions. (Adapted with permission from
APC. (Adapted with permission from Mosnier LO, Zlokovic BV, Griffin JH: The Mosnier LO, Zlokovic BV, Griffin JH: The cytoprotective protein C pathway.
cytoprotective protein C pathway. Blood 109(8):3161–3172, 2007.) Blood 109(8):3161–3172, 2007.)
Kaushansky_chapter 114_p1949-1966.indd 1950 9/18/15 10:05 AM