Page 1977 - Williams Hematology ( PDFDrive )
P. 1977
1952 Part XII: Hemostasis and Thrombosis Chapter 114: Control of Coagulation Reactions 1953
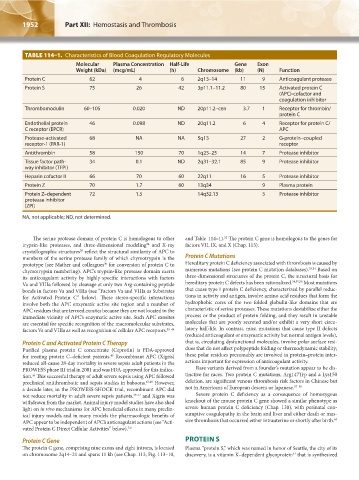
TABLE 114–1. Characteristics of Blood Coagulation Regulatory Molecules
Molecular Plasma Concentration Half-Life Gene Exon
Weight (kDa) (mcg/mL) (h) Chromosome (kb) (N) Function
Protein C 62 4 6 2q13–14 11 9 Anticoagulant protease
Protein S 75 26 42 3p11.1–11.2 80 15 Activated protein C
(APC)-cofactor and
coagulation inhibitor
Thrombomodulin 60–105 0.020 ND 20p11.2–cen 3.7 1 Receptor for thrombin/
protein C
Endothelial protein 46 0.098 ND 20q11.2 6 4 Receptor for protein C/
C receptor (EPCR) APC
Protease-activated 68 NA NA 5q13 27 2 G-protein–coupled
receptor-1 (PAR-1) receptor
Antithrombin 58 150 70 1q23–25 14 7 Protease inhibitor
Tissue factor path- 34 0.1 ND 2q31–32.1 85 9 Protease inhibitor
way inhibitor (TFPI)
Heparin cofactor II 66 70 60 22q11 16 5 Protease inhibitor
Protein Z 70 1.7 60 13q34 9 Plasma protein
Protein Z–dependent 72 1.5 14q32.13 5 Protease inhibitor
protease inhibitor
(ZPI)
NA, not applicable; ND, not determined.
52
The serine protease domain of protein C is homologous to other and Table 114–1). The protein C gene is homologous to the genes for
34
trypsin-like proteases, and three-dimensional modeling and X-ray factors VII, IX, and X (Chap. 113).
30
crystallographic structures reflect the structural similarity of APC to
members of the serine protease family of which chymotrypsin is the Protein C Mutations
30
prototype (see Mather and colleagues for conversion of protein C to Hereditary protein C deficiency associated with thrombosis is caused by
chymotrypsin numbering). APC’s trypsin-like protease domain exerts numerous mutations (see protein C mutation databases). 53,54 Based on
its anticoagulant activity by highly specific interactions with factors three-dimensional structures of the protein C, the structural basis for
Va and VIIIa followed by cleavage at only two Arg-containing peptide hereditary protein C defects has been rationalized. 34,55,56 Most mutations
bonds in factors Va and VIIIa (see “Factors Va and VIIIa as Substrates that cause type I protein C deficiency, characterized by parallel reduc-
for Activated Protein C” below). These stereo-specific interactions tions in activity and antigen, involve amino acid residues that form the
involve both the APC enzymatic active site region and a number of hydrophobic cores of the two folded globulin-like domains that are
APC residues that are termed exosites because they are not located in the characteristic of serine proteases. These mutations destabilize either the
immediate vicinity of APC’s enzymatic active site. Such APC exosites process or the product of protein folding, and they result in unstable
are essential for specific recognition of the macromolecular substrates, molecules that are poorly secreted and/or exhibit a very short circu-
factors Va and VIIIa as well as recognition of cellular APC receptors. 35–44 latory half-life. In contrast, most mutations that cause type II defects
(reduced anticoagulant or enzymatic activity but normal antigen levels),
Protein C and Activated Protein C Therapy that is, circulating dysfunctional molecules, involve polar surface resi-
Purified plasma protein C concentrate (Ceprotin) is FDA-approved dues that do not affect polypeptide folding or thermodynamic stability;
45
for treating protein C–deficient patients. Recombinant APC (Xigris) these polar residues presumably are involved in protein–protein inter-
reduced all-cause 28-day mortality in severe sepsis adult patients in the actions important for expression of anticoagulant activity.
PROWESS phase III trial in 2001 and was FDA-approved for this indica- Rare variants derived from a founder’s mutation appear to be dis-
46
tion. This successful therapy of adult severe sepsis using APC followed tinctive for races. Two protein C mutations, Arg147Trp and a Lys150
preclinical antithrombotic and sepsis studies in baboons. 47,48 However, deletion, are significant venous thrombosis risk factors in Chinese but
a decade later, in the PROWESS-SHOCK trial, recombinant APC did not in Americans of European descent or Japanese. 57–59
not reduce mortality in adult severe sepsis patients, 49–51 and Xigris was Severe protein C deficiency as a consequence of homozygous
withdrawn from the market. Animal injury model studies have also shed knockout of the mouse protein C gene showed a similar phenotype as
light on in vivo mechanisms for APC beneficial effects in many preclin- severe human protein C deficiency (Chap. 130), with perinatal con-
ical injury models and in many models the pharmacologic benefits of sumptive coagulopathy in the brain and liver and either death or mas-
APC appear to be independent of APC’s anticoagulant actions (see “Acti- sive thrombosis that occurred either intrauterine or shortly after birth. 60
vated Protein C Direct Cellular Activities” below). 7,8
Protein C Gene PROTEIN S
The protein C gene, comprising nine exons and eight introns, is located Plasma “protein S,” which was named in honor of Seattle, the city of its
on chromosome 2q14–21 and spans 11 kb (see Chap. 113, Fig. 113–10, discovery, is a vitamin K–dependent glycoprotein that is synthesized
61
Kaushansky_chapter 114_p1949-1966.indd 1952 9/18/15 10:05 AM