Page 2160 - Williams Hematology ( PDFDrive )
P. 2160
2134 Part XII: Hemostasis and Thrombosis Chapter 124: Inherited Deficiencies of Coagulation Factors II, V, V+VIII, VII, X, XI, and XIII 2135
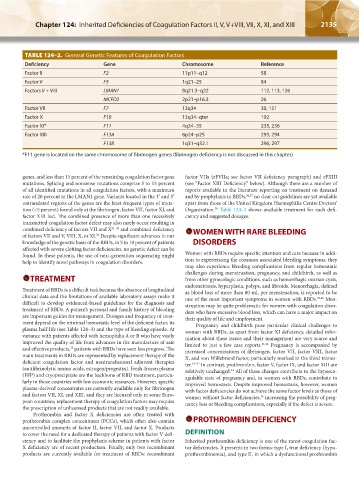
TABLE 124–2. General Genetic Features of Coagulation Factors
Deficiency Gene Chromosome Reference
Factor II F2 11p11–q12 58
Factor V F5 1q21–25 84
Factors V + VIII LMAN1 8q21.3–q22 112, 113, 126
MCFD2 2p21–p16.3 26
Factor VII F7 13q34 30, 151
Factor X F10 13q34–qter 192
Factor XI* F11 4q34–35 235, 236
Factor XIII F13A 6p24–p25 293, 294
F13B 1q31–q32.1 296, 297
*F11 gene is located on the same chromosome of fibrinogen genes (fibrinogen deficiency is not discussed in this chapter)
genes, and less than 15 percent of the remaining coagulation factor gene factor VIIa (rFVIIa; see factor VII deficiency paragraph) and rFXIII
mutations. Splicing and nonsense mutations comprise 5 to 15 percent (see “Factor XIII Deficiency” below). Although there are a number of
of all identified mutations in all coagulation factors, with a maximum reports available in the literature reporting on treatment on demand
rate of 20 percent in the LMAN1 gene. Variants located in the 3′ and 5′ and by prophylaxis in RBDs, 36,37 no clear cut guidelines are yet available
untranslated regions of the genes are the least-frequent types of muta- apart from those of the United Kingdom Haemophilia Centre Doctors’
tion (<5 percent) found only at the fibrinogen, factor VII, factor XI, and Organization. Table 124–3 shows available treatment for each defi-
38
factor XIII loci. The combined presence of more than one recessively ciency and suggested dosages.
transmitted coagulation factor defect may also rarely occur resulting in
combined deficiency of factors VII and X 31–33 and combined deficiency WOMEN WITH RARE BLEEDING
of factors VII and V, VIII, X, or XI. Despite significant advances in our
34
knowledge of the genetic basis of the RBDs, in 5 to 10 percent of patients DISORDERS
affected with severe clotting factor deficiencies, no genetic defect can be
found. In these patients, the use of next-generation sequencing might Women with RBDs require specific attention and care because in addi-
help to identify novel pathways in coagulation disorders. tion to experiencing the common associated bleeding symptoms, they
may also experience bleeding complications from regular hemostatic
challenges during menstruation, pregnancy, and childbirth, as well as
TREATMENT from other gynecologic conditions, such as hemorrhagic ovarian cysts,
endometriosis, hyperplasia, polyps, and fibroids. Menorrhagia, defined
Treatment of RBDs is a difficult task because the absence of longitudinal as blood loss of more than 80 mL per menstruation, is reported to be
clinical data and the limitations of available laboratory assays make it one of the most important symptoms in women with RBDs. 39,40 Men-
difficult to develop evidenced-based guidelines for the diagnosis and struation may be quite problematic for women with coagulation disor-
treatment of RBDs. A patient’s personal and family history of bleeding ders who have excessive blood loss, which can have a major impact on
are important guides for management. Dosages and frequency of treat- their quality of life and employment.
ment depend on the minimal hemostatic level of the deficient factor, its Pregnancy and childbirth pose particular clinical challenges to
plasma half-life (see Table 124–3) and the type of bleeding episode. At women with RBDs, as apart from factor XI deficiency, detailed infor-
variance with patients affected with hemophilia A or B who have vastly mation about these issues and their management are very scarce and
improved the quality of life from advances in the manufacture of safe limited to just a few case reports. 41,42 Pregnancy is accompanied by
and effective products, patients with RBDs have seen less progress. The increased concentrations of fibrinogen, factor VII, factor VIII, factor
35
main treatments in RBDs are represented by replacement therapy of the X, and von Willebrand factor, particularly marked in the third trimes-
deficient coagulation factor and nontransfusional adjuvant therapies ter. 43–47 In contrast, prothrombin, factor V, factor IX, and factor XIII are
(antifibrinolytic amino acids, estrogen/progestin). Fresh-frozen plasma relatively unchanged. All of these changes contribute to the hyperco-
43
(FFP) and cryoprecipitate are the backbone of RBD treatment, particu- agulable state of pregnancy and, in women with RBDs, contribute to
larly in those countries with low economic resources. However, specific improved hemostasis. Despite improved hemostasis, however, women
plasma-derived concentrates are currently available only for fibrinogen with factor deficiencies do not achieve the same factor levels as those of
and factors VII, XI, and XIII, and they are licensed only in some Euro- women without factor deficiencies, increasing the possibility of preg-
39
pean countries; replacement therapy of coagulation factors may require nancy loss or bleeding complications, especially if the defect is severe.
the prescription of unlicensed products that are not readily available.
Prothrombin and factor X deficiencies are often treated with
prothrombin complex concentrates (PCCs), which often also contain PROTHROMBIN DEFICIENCY
uncontrolled amounts of factor II, factor VII, and factor X. Products
to cover the need for a dedicated therapy of patients with factor V defi- DEFINITION
ciency and to facilitate the prophylaxis scheme in patients with factor Inherited prothrombin deficiency is one of the rarest coagulation fac-
X deficiency are of recent production. Finally, only two recombinant tor deficiencies. It presents in two forms: type I, true deficiency (hypo-
products are currently available for treatment of RBDs: recombinant prothrombinemia), and type II, in which a dysfunctional prothrombin
Kaushansky_chapter 124_p2133-2150.indd 2135 17/09/15 3:40 pm