Page 2177 - Williams Hematology ( PDFDrive )
P. 2177
2152 Part XII: Hemostasis and Thrombosis Chapter 125: Hereditary Fibrinogen Abnormalities 2153
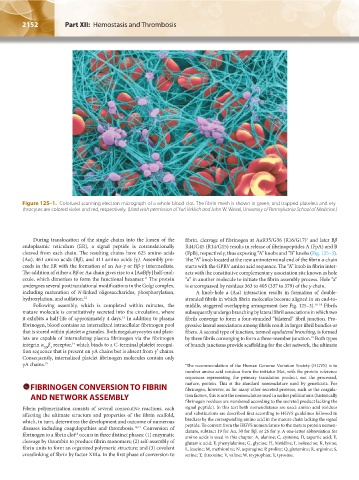
Figure 125–1. Colorized scanning electron micrograph of a whole blood clot. The fibrin mesh is shown in green, and trapped platelets and ery-
throcytes are colored violet and red, respectively. (Used with permission of Yuri Veklich and John W. Weisel, University of Pennsylvania School of Medicine.)
During translocation of the single chains into the lumen of the fibrin, cleavage of fibrinogen at AαR35/G36 (R16/G17) and later Bβ
*
endoplasmic reticulum (ER), a signal peptide is cotranslationally R44/G45 (R14/G15) results in release of fibrinopeptides A (FpA) and B
cleaved from each chain. The resulting chains have 625 amino acids (FpB), respectively, thus exposing “A” knobs and “B” knobs (Fig. 125–3).
(Aα), 461 amino acids (Bβ), and 411 amino acids (γ). Assembly pro- The “A” knob located at the new aminoterminal end of the fibrin α chain
ceeds in the ER with the formation of an Aα-γ or Bβ-γ intermediate. starts with the GPRV amino acid sequence. The “A” knob in fibrin inter-
The addition of either a Bβ or Aα chain gives rise to a [AαBβγ] half-mol- acts with the constitutive complementary association site known as hole
11
ecule, which dimerizes to form the functional hexamer. The protein “a” in another molecule to initiate the fibrin assembly process. Hole “a”
undergoes several posttranslational modifications in the Golgi complex, is encompassed by residues 363 to 405 (337 to 379) of the γ chain.
including maturation of N-linked oligosaccharides, phosphorylation, A knob-hole a (A:a) interaction results in formation of double-
hydroxylation, and sulfation. 12 stranded fibrils in which fibrin molecules become aligned in an end-to-
Following assembly, which is completed within minutes, the middle, staggered overlapping arrangement (see Fig. 125–3). 16–18 Fibrils
mature molecule is constitutively secreted into the circulation, where subsequently undergo branching by lateral fibril associations in which two
13
it exhibits a half-life of approximately 4 days. In addition to plasma fibrils converge to form a four-stranded “bilateral” fibril junction. Pro-
fibrinogen, blood contains an internalized intracellular fibrinogen pool gressive lateral associations among fibrils result in larger fibril bundles or
that is stored within platelet α granules. Both megakaryocytes and plate- fibers. A second type of junction, termed equilateral branching, is formed
lets are capable of internalizing plasma fibrinogen via the fibrinogen by three fibrils converging to form a three-member junction. Both types
19
integrin α β receptor, which binds to a C-terminal platelet recogni- of branch junctions provide scaffolding for the clot network, the ultimate
14
IIb 3
tion sequence that is present on γA chains but is absent from γ′ chains.
Consequently, internalized platelet fibrinogen molecules contain only
γA chains. 15 * The recommendation of the Human Genome Variation Society (HGVS) is to
number amino acid residues from the initiator Met, with the protein reference
sequences representing the primary translation product, not the processed,
FIBRINOGEN CONVERSION TO FIBRIN mature, protein. This is the standard nomenclature used by geneticists. For
fibrinogen, however, as for many other secreted proteins, such as the coagula-
AND NETWORK ASSEMBLY tion factors, this is not the nomenclature used in earlier publications (historically
fibrinogen residues are numbered according to the secreted product lacking the
Fibrin polymerization consists of several consecutive reactions, each signal peptide). In this text both nomenclatures are used: amino acid residues
affecting the ultimate structure and properties of the fibrin scaffold, and substitutions are described first according to HGVS guidelines followed in
which, in turn, determines the development and outcome of numerous brackets by the corresponding amino acid in the mature chain lacking the signal
diseases including coagulopathies and thrombosis. 16,17 Conversion of peptide. To convert from the HGVS nomenclature to the mature protein nomen-
clature, subtract 19 for Aα, 30 for Bβ, or 26 for γ. A one-letter abbreviation for
18
fibrinogen to a fibrin clot occurs in three distinct phases: (1) enzymatic amino acids is used in this chapter. A, alanine; C, cysteine; D, aspartic acid; E,
cleavage by thrombin to produce fibrin monomers; (2) self-assembly of glutamic acid; F, phenylalanine; G, glycine; H, histidine; I, isoleucine; K, lysine;
fibrin units to form an organized polymeric structure; and (3) covalent L, leucine; M, methionine; N, asparagine; P, proline; Q, glutamine; R, arginine; S,
crosslinking of fibrin by factor XIIIa. In the first phase of conversion to serine; T, threonine; V, valine; W, tryptophan; Y, tyrosine.
Kaushansky_chapter 125_p2151-2162.indd 2152 9/18/15 5:47 PM