Page 2178 - Williams Hematology ( PDFDrive )
P. 2178
2152 Part XII: Hemostasis and Thrombosis Chapter 125: Hereditary Fibrinogen Abnormalities 2153
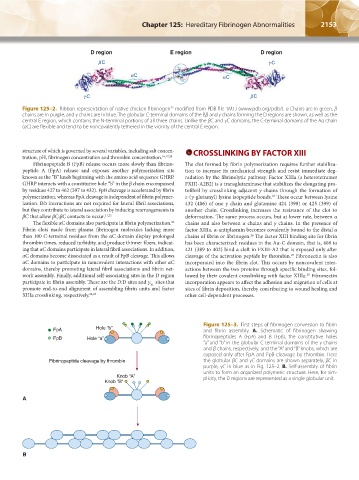
Figure 125–2. Ribbon representation of native chicken fibrinogen modified from PDB file 1M1J (www.pdb.org/pdb/). α Chains are in green, β
22
chains are in purple, and γ chains are in blue. The globular C-terminal domains of the Bβ and γ chains forming the D regions are shown, as well as the
central E region, which contains the N-terminal portions of all three chains. Unlike the βC and γC domains, the C-terminal domains of the Aα chain
(αC) are flexible and tend to be noncovalently tethered in the vicinity of the central E region.
structure of which is governed by several variables, including salt concen- CROSSLINKING BY FACTOR XIII
tration, pH, fibrinogen concentration and thrombin concentration. 16,17,20
Fibrinopeptide B (FpB) release occurs more slowly than fibrino- The clot formed by fibrin polymerization requires further stabiliza-
peptide A (FpA) release and exposes another polymerization site tion to increase its mechanical strength and resist immediate deg-
known as the “B” knob beginning with the amino acid sequence GHRP. radation by the fibrinolytic pathway. Factor XIIIa (a heterotetramer
GHRP interacts with a constitutive hole “b” in the β chain encompassed FXIII-A2B2) is a transglutaminase that stabilizes the elongating pro-
by residues 427 to 462 (397 to 432). FpB cleavage is accelerated by fibrin tofibril by crosslinking adjacent γ chains through the formation of
polymerization, whereas FpA cleavage is independent of fibrin polymer- ε-(γ-glutamyl) lysine isopeptide bonds. These occur between lysine
26
ization. B:b interactions are not required for lateral fibril associations, 432 (406) of one γ chain and glutamine 424 (398) or 425 (399) of
but they contribute to lateral association by inducing rearrangements in another chain. Crosslinking increases the resistance of the clot to
βC that allow βC:βC contacts to occur. 21,22 deformation. The same process occurs, but at lower rate, between α
The flexible αC domains also participate in fibrin polymerization. chains and also between α chains and γ chains. In the presence of
23
Fibrin clots made from plasma fibrinogen molecules lacking more factor XIIIa, α-antiplasmin becomes covalently bound to the distal α
than 100 C-terminal residues from the αC domain display prolonged chains of fibrin or fibrinogen. The factor XIII binding site for fibrin
26
thrombin times, reduced turbidity, and produce thinner fibers, indicat- has been characterized: residues in the Aα-C domain, that is, 408 to
ing that αC domains participate in lateral fibril associations. In addition, 421 (389 to 402) bind a cleft in FXIII-A2 that is exposed only after
αC domains become dissociated as a result of FpB cleavage. This allows cleavage of the activation peptide by thrombin. Fibronectin is also
27
αC domains to participate in noncovalent interactions with other αC incorporated into the fibrin clot. This occurs by noncovalent inter-
domains, thereby promoting lateral fibril associations and fibrin net- actions between the two proteins through specific binding sites, fol-
work assembly. Finally, additional self-associating sites in the D region lowed by their covalent crosslinking with factor XIIIa. Fibronectin
28
participate in fibrin assembly. These are the D:D sites and γ sites that incorporation appears to affect the adhesion and migration of cells at
XL
promote end-to-end alignment of assembling fibrin units and factor sites of fibrin deposition, thereby contributing to wound healing and
XIIIa crosslinking, respectively. 24,25 other cell-dependent processes.
Figure 125–3. First steps of fibrinogen conversion to fibrin
FpA Hole “b” and fibrin assembly. A. Schematic of fibrinogen showing
FpB Hole “a” fibrinopeptides A (FpA) and B (FpB), the constitutive holes
“a” and “b” in the globular C-terminal domains of the γ chains
and β chains, respectively, and the “A” and “B” knobs, which are
exposed only after FpA and FpB cleavage by thrombin. Here
Fibrinopeptide cleavage by thrombin the globular βC and γC domains are shown separately, βC in
purple, γC in blue as in Fig. 125–2. B. Self-assembly of fibrin
units to form an organized polymeric structure. Here, for sim-
Knob “A” plicity, the D regions are represented as a single globular unit.
Knob “B”
A
B
Kaushansky_chapter 125_p2151-2162.indd 2153 9/18/15 5:47 PM