Page 2182 - Williams Hematology ( PDFDrive )
P. 2182
2156 Part XII: Hemostasis and Thrombosis Chapter 125: Hereditary Fibrinogen Abnormalities 2157
In addition to fibrinogen substitution, antifibrinolytic agents may many different mutations in the three fibrinogen-encoding genes. Dys-
be given, particularly to treat mucosal bleeding or to prevent bleeding fibrinogenemias and hypodysfibrinogenemias are autosomal dominant
following procedures such as dental extraction. Fibrin glue is useful to disorders. Most affected patients are heterozygous for missense muta-
treat superficial wounds or following dental extractions. Oral contra- tions in the coding region of one of the three fibrinogen genes. Because
ceptive preparations are useful in case of menorrhagia. Oral iron prep- the secreted fibrinogen hexamer contains two copies of each of the three
arations can be given in cases with associated iron-deficiency anemia. fibrinogen chains, and the resulting fibrin network contains multiple cop-
Routine vaccination against hepatitis, as well as a regular surveillance ies of the molecule, heterozygosity for one mutant allele is sufficient to
for both the disease and treatment-related complications in a compre- impair the structure and function of the fibrin clot (Fig. 125–4).
hensive care setting, is highly recommended. 64
Finally, orthotopic liver transplantation is a possible rescue treat-
ment for failure of fibrinogen replacement therapy. This procedure suc-
cessfully restored normal hemostasis in an afibrinogenemic patient with
71
severe Budd-Chiari syndrome and inferior cava vein thrombosis and
in one of the four afibrinogenemic patients homozygous for the 11-kb
FGA mutation. 35,72
Complications of Therapy
In many countries only FFP or cryoprecipitate are available, which is
problematic because the viral inactivation process is in general not as
efficient as it is for fibrinogen concentrates (although emerging non-
viral pathogens such as the prion responsible for variant Creutzfeldt-
Jacob disease must be considered, even for concentrates). Even if viral
inactivation steps are performed, these preparations (particularly FFP)
can induce volume overload. There is also a risk of transfusion-related
acute lung injury, because of the presence of cytotoxic antibodies in the
infused plasma.
Acquired inhibitors to fibrinogen after replacement therapy have
been reported in only two cases. It is not clear why afibrinogenemic
patients do not develop inhibitors more frequently. One explana-
tion for some cases is that minute amounts of fibrinogen, which can
only be detected by highly sensitive immunoassays, are present in the
circulation.
One of the major complications in afibrinogenemic patients is
thrombosis, which can occur spontaneously following blood com-
ponent therapy. Some clinicians give small doses of heparin or low-
molecular-weight heparin (LMWH) during administration of fibrin-
ogen. Before surgery, patients with a thrombotic phenotype should
be treated with compression stockings and LMWH. Successful use of
lepirudin has been reported for an afibrinogenemic patient who suf-
fered recurrent arterial thrombosis despite treatment with heparin
and aspirin. Thromboembolic complications are difficult to manage
73
because both anticoagulants and fibrinogen preparations have to be
administered.
New Preparations
The increasing need for fibrinogen preparations in congenital but also
in acquired deficiencies has stimulated some companies to improve
existing preparations or to develop new ones. A recombinant fibrinogen
molecule is also under development. 74
DYSFIBRINOGENEMIA AND
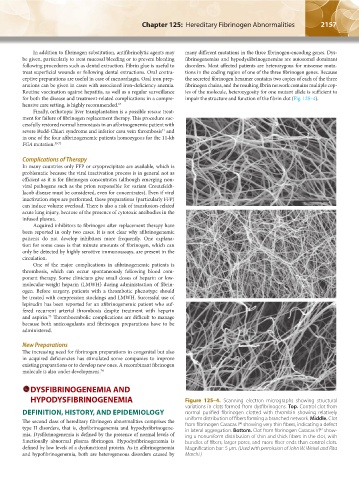
HYPODYSFIBRINOGENEMIA Figure 125–4. Scanning electron micrographs showing structural
variations in clots formed from dysfibrinogens. Top. Control clot from
DEFINITION, HISTORY, AND EPIDEMIOLOGY normal purified fibrinogen clotted with thrombin showing relatively
The second class of hereditary fibrinogen abnormalities comprises the uniform distribution of fibers forming a branched network. Middle. Clot
96
type II disorders, that is, dysfibrinogenemia and hypodysfibrinogene- from fibrinogen Caracas I showing very thin fibers, indicating a defect
97
in lateral aggregation. Bottom. Clot from fibrinogen Caracas VI show-
mia. Dysfibrinogenemia is defined by the presence of normal levels of ing a nonuniform distribution of thin and thick fibers in the clot, with
functionally abnormal plasma fibrinogen. Hypodysfibrinogenemia is bundles of fibers, larger pores, and more fiber ends than control clots.
defined by low levels of a dysfunctional protein. As in afibrinogenemia Magnification bar: 5 μm. (Used with permission of John W. Weisel and Rita
and hypofibrinogenemia, both are heterogeneous disorders caused by Marchi.)
Kaushansky_chapter 125_p2151-2162.indd 2157 9/18/15 5:48 PM