Page 348 - Williams Hematology ( PDFDrive )
P. 348
322 Part V: Therapeutic Principles Chapter 22: Pharmacology and Toxicity of Antineoplastic Drugs 323
O
C N
N
S S N
C N C N NH 2 N
N N
N N
N NH 2 N O
HO CH 2
OH
OH
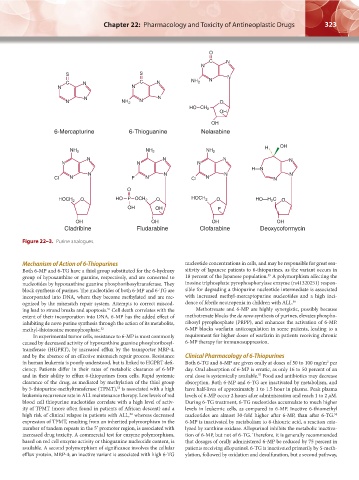
6-Mercapturine 6-Thioguanine Nelarabine
H OH
NH 2 NH 2 NH 2
N N N N
N N N
H—N
N N N N
Cl N F N Cl N N
O
HOCH 2 O HO P OCH 2 O HOCH 2 O HO—H C O
2
OH OH F
OH OH OH OH
Cladribine Fludarabine Clofarabine Deoxycoformycin
Figure 22–3. Purine analogues.
Mechanism of Action of 6-Thiopurines nucleotide concentrations in cells, and may be responsible for great sen-
Both 6-MP and 6-TG have a thiol group substituted for the 6-hydroxy sitivity of Japanese patients to 6-thiopurines, as the variant occurs in
55
group of hypoxanthine or guanine, respectively, and are converted to 18 percent of the Japanese population. A polymorphism affecting the
nucleotides by hypoxanthine guanine phosphoribosyltransferase. They inosine triphosphate pyrophosphorylase enzyme (rs41320251) respon-
block synthesis of purines. The nucleotides of both 6-MP and 6-TG are sible for degrading a thiopurine nucleotide intermediate is associated
incorporated into DNA, where they become methylated and are rec- with increased methyl-mercaptopurine nucleotides and a high inci-
ognized by the mismatch repair system. Attempts to correct miscod- dence of febrile neutropenia in children with ALL. 56
51
ing lead to strand breaks and apoptosis. Cell death correlates with the Methotrexate and 6-MP are highly synergistic, possibly because
extent of their incorporation into DNA. 6-MP has the added effect of methotrexate blocks the de novo synthesis of purines, elevates phospho-
inhibiting de novo purine synthesis through the action of its metabolite, ribosyl pyrophosphate (PRPP), and enhances the activation of 6-MP.
methyl-thioinosine monophosphate. 52 6-MP blocks warfarin anticoagulation in some patients, leading to a
In experimental tumor cells, resistance to 6-MP is most commonly requirement for higher doses of warfarin in patients receiving chronic
caused by decreased activity of hypoxanthine guanine phosphoribosyl- 6-MP therapy for immunosuppression.
transferase (HGPRT), by increased efflux by the transporter MRP-4,
and by the absence of an effective mismatch repair process. Resistance Clinical Pharmacology of 6-Thiopurines
in human leukemia is poorly understood, but is linked to HGPRT defi- Both 6-TG and 6-MP are given orally at doses of 50 to 100 mg/m per
2
ciency. Patients differ in their rates of metabolic clearance of 6-MP day. Oral absorption of 6-MP is erratic, as only 16 to 50 percent of an
and in their ability to efflux 6-thiopurines from cells. Rapid systemic oral dose is systemically available. Food and antibiotics may decrease
57
clearance of the drug, as mediated by methylation of the thiol group absorption. Both 6-MP and 6-TG are inactivated by metabolism, and
by 5-thiopurine-methyltransferase (TPMT), is associated with a high have half-lives of approximately 1 to 1.5 hour in plasma. Peak plasma
53
leukemia recurrence rate in ALL maintenance therapy. Low levels of red levels of 6-MP occur 2 hours after administration and reach 1 to 2 μM.
blood cell thiopurine nucleotides correlate with a high level of activ- During 6-TG treatment, 6-TG nucleotides accumulate to much higher
ity of TPMT (more often found in patients of African descent) and a levels in leukemic cells, as compared to 6-MP. Inactive 6-thiomethyl
high risk of clinical relapse in patients with ALL, whereas decreased nucleotides are almost 30-fold higher after 6-MP, than after 6-TG.
54
58
expression of TPMT, resulting from an inherited polymorphism in the 6-MP is inactivated by metabolism to 6-thiouric acid, a reaction cata-
number of tandem repeats in the 5′ promoter region, is associated with lyzed by xanthine oxidase. Allopurinol inhibits the metabolic inactiva-
increased drug toxicity. A commercial test for enzyme polymorphism, tion of 6-MP, but not of 6-TG. Therefore, it is generally recommended
based on red cell enzyme activity or thioguanine nucleotide content, is that dosages of orally administered 6-MP be reduced by 75 percent in
available. A second polymorphism of significance involves the cellular patients receiving allopurinol. 6-TG is inactivated primarily by S-meth-
efflux protein, MRP-4; an inactive variant is associated with high 6-TG ylation, followed by oxidation and desulfuration, but a second pathway,
Kaushansky_chapter 22_p0313-0352.indd 323 9/18/15 10:24 PM