Page 35 - Williams Hematology ( PDFDrive )
P. 35
12 Part I: Clinical Evaluation of the Patient Chapter 2: Examination of Blood Cells 13
analytical challenges are the frequency of the different cell types, which as basophils and immature granulocytic cells, from the major normal
vary over many orders of magnitude, from red cells (millions per μL) to blood cell types. In addition, nucleic-acid-binding fluorescent dyes incor-
basophils (dozens per μL), and the complexity of the structure of normal porated into the lysis buffer measure total RNA plus DNA in the cells and
and abnormal blood cells. Over the past several decades, instruments are used in some analyzers to help differentiate leukocyte types. Fluores-
have become increasingly sophisticated with the use of multiple param- cence measurements after staining with RNA binding dyes are commonly
eters to produce more precise results in the great majority of patient used to detect and subclassify reticulocytes and platelets. Light absorp-
samples. In a typical automated hematology analyzer, the blood sample tion is the principle used for hemoglobin measurement and in some
is aspirated and separated into different fluidic streams. The streams instruments for identifying peroxidase-positive granulocytes. Instru-
are mixed with various buffers that accomplish specific purposes in ments rely on a combination of techniques for accuracy and precision
11
the analysis, for instance, using differential lysis to distinguish subsets (Fig. 2–1). Complex algorithms are invoked to determine whether the
of leukocytes, reagents to measure hemoglobin or detect myeloperoxi- distribution of variables for a specific result or for the specimen as a whole
dase containing leukocytes, and various fluorescent dyes. Measurements fit sufficiently within an expected variable space so that the results can
of each fluidic stream are made in flow as the sample passes through be reported with high confidence, or whether the specimen should be
a series of detectors in what are essentially modified flow cytometers “flagged” for further analysis or manual blood film review (Fig. 2–2). There
(Chap. 3). Commonly used principles include light scatter at various is significant overlap in methodology between automated hematology
angles, electrical impedance and conductivity, and fluorescence or light analyzers and flow cytometers (flow cytometers are discussed in Chap. 3).
absorption of cells stained in flow. Light scatter yields information about The latter are distinguished by extensive use of fluorochrome tagged anti-
cell size (using scatter at low-incident angles), nuclear lobulation, and bodies to identify cell subtypes. These instruments have replaced labori-
cytoplasmic granularity (using high-angle light scatter) and refractive ous manual work, but also demand increasing interpretation skills on the
index, with polarization of the scattered light as an additional param- part of laboratory technologists. Automated blood analyzers have been
eter. If red cells are converted to spherocytes by the buffer solution to adapted to accurately count the smaller numbers of blood cells typically
13
12
eliminate the variability of cell shape, light scatter at different angles found in body fluids, but accurate differential counts and detection of
14
can provide information about hemoglobin content, as well as size of blast cells in fluids of patients remains a challenge.
individual red cells. Cell size is also estimated by measuring change in Point of care “bedside” testing is far more challenging in hematol-
electrical resistance, which is proportional to cell size as cells enter a ogy than for typical clinical chemistry analytes for many of the reasons
narrow orifice through which a direct current is maintained, the orig- described above. Instruments have been described for bedside measure-
inal Coulter principle, named for Wallace Coulter who developed the ment of hemoglobin, total leukocytes, three-part leukocyte differential
10
electronic particle counter. Radiofrequency capacitance measurement count, malaria parasitemia, and CD4+ T-cell count, mainly targeting
yields additional intracellular structural information that complements clinical settings with limited access to standard laboratory testing. More
the direct current measurement. Differential lysis with detergents of work remains to be done to demonstrate the reliability and clinical
varying strength or pH is used to separate certain leukocyte types, such impact of such testing strategies. 15
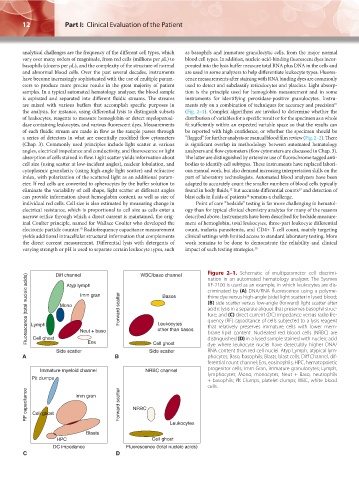
Figure 2–1. Schematic of multiparameter cell discrimi-
Diff channel
WBC/baso channel
Fluorescence (total nucleic acids) Lymph Mono Imm gran Forward scatter Leukocytes XE-2100 is used as an example, in which leukocytes are dis-
nation in an automated hematology analyzer. The Sysmex
Atyp lymph
criminated by (A) DNA/RNA fluorescence using a polyme-
Basos
thine dye versus high-angle (side) light scatter in lysed blood;
(B) side scatter versus low-angle (forward) light scatter after
acidic lysis in a separate aliquot that preserves basophil struc-
ture; and (C) direct current (DC) impedance versus radio fre-
quency (RF) capacitance of cells subjected to a lysis reagent
that relatively preserves immature cells with lower mem-
other than basos
Cell ghost
distinguished (D) in a lysed sample stained with nucleic acid
Eos
Cell ghost
dye where leukocyte nuclei have detectably higher DNA/
RNA content than red cell nuclei. Atyp Lymph, atypical lym-
Side scatter Neut + baso Side scatter brane lipid content. Nucleated red blood cells (NRBC) are
A B phocytes; Baso, basophils; Blasts, blast cells; Diff Channel, dif-
ferential count channel; Eos, eosinophils; HPC, hematopoietic
Immature myeloid channel NRBC channel progenitor cells; Imm Gran, immature granulocytes; Lymph,
lymphocytes; Mono, monocytes; Neut + Baso, neutrophils
Pit clumps + basophils; Plt Clumps, platelet clumps; WBC, white blood
cells.
RF capacitance Cell ghost Imm gran Forward scatter NRBC
Leukocytes
Blasts
HPC Cell ghost
DC impedance Fluorescence (total nucleic acids)
C D
Kaushansky_chapter 02_p0011-0026.indd 12 17/09/15 5:34 pm