Page 463 - Williams Hematology ( PDFDrive )
P. 463
438 Part V: Therapeutic Principles Chapter 29: Gene Therapy for Hematologic Diseases 439
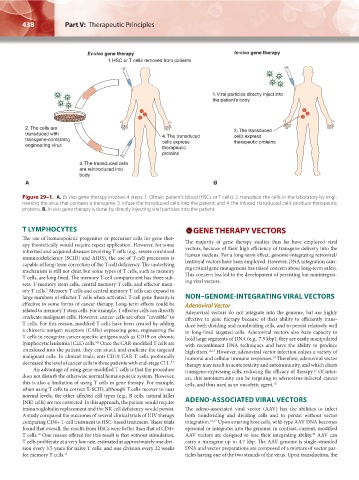
Ex-vivo gene therapy In-vivo gene therapy
1. HSC or T cells removed from patients
1. Viral particles directly inject into
the patient’s body
2. The cells are 2. The transduced
transduced with 4. The transduced cells express
transgene-containing cells express therapeutic proteins
engineering virus therapeutic
proteins
3. The transduced cells
are reintroduced into
body
A B
Figure 29–1. A. Ex vivo gene therapy involves 4 steps: 1. Obtain patient’s blood (HSCs or T cells); 2. transduce the cells in the laboratory by engi-
neering the virus that contains a transgene; 3. infuse the transduced cells into the patient; and 4. the infused transduced cells produce therapeutic
proteins. B. In vivo gene therapy is done by directly injecting viral particles into the patient.
T LYMPHOCYTES GENE THERAPY VECTORS
The use of hematopoietic progenitor or precursor cells for gene ther-
apy theoretically would require repeat application. However, for some The majority of gene therapy studies thus far have employed viral
inherited and acquired diseases involving T cells (e.g., severe combined vectors, because of their high efficiency of transgene delivery into the
immunodeficiency [SCID] and AIDS), the use of T-cell precursors is human nucleus. For a long-term effect, genome-integrating retroviral/
capable of long-term correction of the T-cell deficiency. The underlying lentiviral vectors have been employed. However, DNA integration caus-
mechanism is still not clear, but some types of T cells, such as memory ing critical gene mutagenesis has raised concern about long-term safety.
T cells, are long-lived. The memory T-cell compartment has three sub- This concern has led to the development of persisting but nonintegrat-
sets: T-memory stem cells, central memory T cells, and effector mem- ing viral vectors.
ory T cells. Memory T cells and central memory T cells can expand to
7
large numbers of effector T cells when activated. T-cell gene therapy is NON–GENOME-INTEGRATING VIRAL VECTORS
effective in some forms of cancer therapy. Long-term effects could be Adenoviral Vector
related to memory T stem cells. For example, T effector cells can directly Adenoviral vectors do not integrate into the genome, but are highly
eradicate malignant cells. However, cancer cells are often “invisible” to effective in gene therapy because of their ability to efficiently trans-
T cells. For this reason, modified T cells have been created by adding duce both dividing and nondividing cells, and to persist relatively well
a chimeric antigen receptors (CARs) expressing gene, engineering the in long-lived targeted cells. Adenoviral vectors also have capacity to
T cells to recognize cancer-specific antigens such as CD19 on chronic hold large segments of DNA (e.g., 7.5 kbp); they are easily manipulated
lymphocytic leukemia (CLL) cells. Once the CAR-modified T cells are with recombinant DNA techniques and have the ability to produce
8,9
transfused into the patient, they can attack and eradicate the targeted high titers. 12,13 However, adenoviral vector infection enlists a variety of
malignant cells. In clinical trials, anti-CD19 CAR T cells profoundly humoral and cellular immune responses. Therefore, adenoviral vector
14
decreased the level of cancer cells in three patients with end-stage CLL. 8,9 therapy may result in acute toxicity and autoimmunity, and which clears
An advantage of using gene-modified T cells is that the procedure transgene-expressing cells, reducing the efficacy of therapy. Of inter-
13
does not disturb the otherwise normal hematopoietic system. However, est, this autoimmunity can be targeting to adenovirus-infected cancer
this is also a limitation of using T cells in gene therapy. For example, cells, and thus used as an oncolytic agent. 15
when using T cells to correct X-SCID, although T cells recover to near
normal levels, the other affected cell types (e.g., B cells, natural killer
[NK] cells) are not corrected. In this approach, the patient would require ADENO-ASSOCIATED VIRAL VECTORS
immunoglobulin replacement and the NK cell deficiency would persist. The adeno-associated viral vector (AAV) has the abilities to infect
A study compared the outcomes of several clinical trials of HIV therapy both nondividing and dividing cells and to persist without vector
comparing CD4+ T-cell treatment to HSC-based treatment. These trials integration. 16,17 Upon entering host cells, wild-type AAV DNA becomes
found that overall, the results from HSCs were better than that of CD4+ episomal or integrates into the genome; in contrast, current, modified
T cells. One reason offered for this result is that without stimulation, AAV vectors are designed to lose their integrating ability. AAV can
18
10
T cells proliferate at a very low rate, estimated at approximately one divi- carry a transgene up to 4.7 kbp. The AAV genome is single-stranded
sion every 3.5 years for naïve T cells, and one division every 22 weeks DNA and vector preparations are composed of a mixture of vector par-
for memory T cells. 11 ticles having one of the two strands of the virus. Upon transduction, the
Kaushansky_chapter 29_p0437-0446.indd 438 9/19/15 12:22 AM