Page 468 - Williams Hematology ( PDFDrive )
P. 468
442 Part V: Therapeutic Principles Chapter 29: Gene Therapy for Hematologic Diseases 443
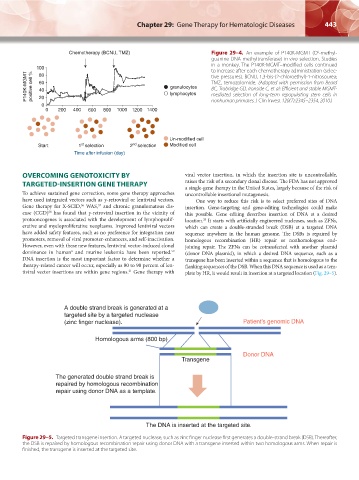
Chemotherapy (BCNU, TMZ) Figure 29–4. An example of P140K-MGMT (O -methyl-
6
guanine-DNA methyltransferase) in vivo selection. Studies
in a monkey. The P140K-MGMT–modified cells continued
100 to increase after each chemotherapy administration (selec-
P140K-MGMT positive cell % 60 Iymphocytes TMZ, temozolomide. (Adapted with permission from Beard
80
tive pressures). BCNU, 1,3-bis-(2-chloroethyl)-1-nitrosourea;
granulocytes
BC, Trobridge GD, Ironside C, et al: Efficient and stable MGMT-
40
mediated selection of long-term repopulating stem cells in
20
0 nonhuman primates. J Clin Invest 120(7):2345–2354, 2010.)
0 200 400 600 800 1000 1200 1400
Un-modified cell
st
Start 1 selection 2 nd selection Modified cell
Time after infusion (day)
OVERCOMING GENOTOXICITY BY viral vector insertion, in which the insertion site is uncontrollable,
TARGETED-INSERTION GENE THERAPY raises the risk of a secondary clonal disease. The FDA has not approved
a single-gene therapy in the United States, largely because of the risk of
To achieve sustained gene correction, some gene therapy approaches uncontrollable insertional mutagenesis.
have used integrated vectors such as γ-retroviral or lentiviral vectors. One way to reduce this risk is to select preferred sites of DNA
Gene therapy for X-SCID, WAS, and chronic granulomatous dis- insertion. Gene-targeting and gene-editing technologies could make
56
57
ease (CGD) has found that γ-retroviral insertion in the vicinity of this possible. Gene editing describes insertion of DNA at a desired
58
protooncogenes is associated with the development of lymphoprolif- location. It starts with artificially engineered nucleases, such as ZFNs,
33
erative and myeloproliferative neoplasms. Improved lentiviral vectors which can create a double-stranded break (DSB) at a targeted DNA
have added safety features, such as no preference for integration near sequence anywhere in the human genome. The DSBs is repaired by
promoters, removal of viral promoter-enhancers, and self-inactivation. homologous recombination (HR) repair or nonhomologous end-
However, even with these new features, lentiviral vector-induced clonal joining repair. The ZFNs can be cotransfected with another plasmid
dominance in human and murine leukemia have been reported. (donor DNA plasmid), in which a desired DNA sequence, such as a
59
6
DNA insertion is the most important factor to determine whether a transgene has been inserted within a sequence that is homologous to the
therapy-related cancer will occur, especially as 80 to 90 percent of len- flanking sequences of the DSB. When this DNA sequence is used as a tem-
tiviral vector insertions are within gene regions. Gene therapy with plate by HR, it would result in insertion at a targeted location (Fig. 29–5).
21
A double strand break is generated at a
targeted site by a targeted nuclease
(zinc finger nuclease). Patient’s genomic DNA
Homologous arms (800 bp)
Donor DNA
Transgene
The generated double strand break is
repaired by homologous recombination
repair using donor DNA as a template.
The DNA is inserted at the targeted site.
Figure 29–5. Targeted transgene insertion. A targeted nuclease, such as zinc finger nuclease first generates a double-strand break (DSB). Thereafter,
the DSB is repaired by homologous recombination repair using donor DNA with a transgene inserted within two homologous arms. When repair is
finished, the transgene is inserted at the targeted site.
Kaushansky_chapter 29_p0437-0446.indd 443 9/19/15 12:22 AM