Page 859 - Williams Hematology ( PDFDrive )
P. 859
834 Part VI: The Erythrocyte Chapter 54: Hemolytic Anemia Resulting from Immune Injury 835
TABLE 54–4. Major Reaction Patterns of the Direct Quantity, Affinity, and Isotype of Red Blood Cell–Bound
Autoantibody
Antiglobulin Test and Associated Types of Immune Injury
Direct Antiglobulin Test–Negative Autoimmune Hemolytic Anemia
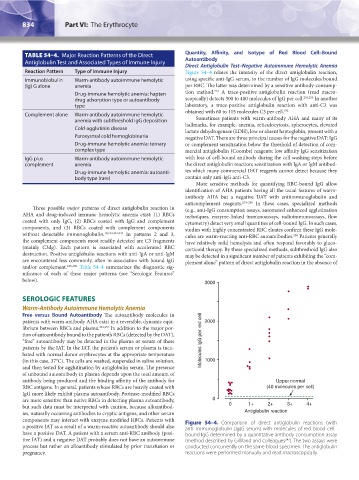
Reaction Pattern Type of Immune Injury Figure 54–4 relates the intensity of the direct antiglobulin reaction,
Immunoblobulin Warm-antibody autoimmune hemolytic using specific anti-IgG serum, to the number of IgG molecules bound
(Ig) G alone anemia per RBC. The latter was determined by a sensitive antibody-consump-
254
Drug-immune hemolytic anemia: hapten tion method. A trace-positive antiglobulin reaction (read macro-
drug adsorption type or autoantibody scopically) detects 300 to 400 molecules of IgG per cell. 254,255 In another
type laboratory, a trace-positive antiglobulin reaction with anti-C3 was
obtained with 60 to 115 molecules C3 per cell. 182
Complement alone Warm-antibody autoimmune hemolytic
anemia with subthreshold IgG deposition Sometimes patients with warm-antibody AHA and many of its
Cold-agglutinin disease hallmarks, for example, anemia, reticulocytosis, spherocytes, elevated
lactate dehydrogenase (LDH), low or absent haptoglobin, present with a
Paroxysmal cold hemoglobinuria negative DAT. There are three principal causes for the negative DAT: IgG
Drug-immune hemolytic anemia: ternary or complement sensitization below the threshold of detection of com-
complex type mercial antiglobulin (Coombs) reagents; low affinity IgG sensitization
IgG plus Warm-antibody autoimmune hemolytic with loss of cell-bound antibody during the cell washing steps before
complement anemia the direct antiglobulin reaction; sensitization with IgA or IgM antibod-
Drug-immune hemolytic anemia: autoanti- ies which many commercial DAT reagents cannot detect because they
body type (rare) contain only anti-IgG anti-C3.
More sensitive methods for quantifying RBC-bound IgG allow
identification of AHA patients having all the usual features of warm-
antibody AHA but a negative DAT with antiimmunoglobulin and
anticomplement reagents. 254–256 In these cases, specialized methods
Three possible major patterns of direct antiglobulin reaction in (e.g., anti-IgG consumption assays, automated enhanced agglutination
AHA and drug-induced immune hemolytic anemia exist: (1) RBCs techniques, enzyme-linked immunoassays, radioimmunoassays, flow
coated with only IgG, (2) RBCs coated with IgG and complement cytometry) detect very small quantities of cell-bound IgG. In such cases,
components, and (3) RBCs coated with complement components studies with highly concentrated RBC eluates confirm these IgG mole-
without detectable immunoglobulin. 10,123,243,244 In patterns 2 and 3, cules are warm-reacting anti-RBC autoantibodies. Patients generally
254
the complement components most readily detected are C3 fragments have relatively mild hemolysis and often respond favorably to gluco-
(mainly C3dg). Each pattern is associated with accelerated RBC corticoid therapy. By these specialized methods, subthreshold IgG also
destruction. Positive antiglobulin reactions with anti-IgA or anti-IgM may be detected in a significant number of patients exhibiting the “com-
are encountered less commonly, often in association with bound IgG plement alone” pattern of direct antiglobulin reaction in the absence of
and/or complement. 245–251 Table 54–4 summarizes the diagnostic sig-
nificance of each of these major patterns (see “Serologic Features”
below).
3000
SEROLOGIC FEATURES
Warm-Antibody Autoimmune Hemolytic Anemia
Free versus Bound Autoantibody The autoantibody molecules in
patients with warm-antibody AHA exist in a reversible, dynamic equi- 2000
librium between RBCs and plasma. 252,253 In addition to the major por-
tion of autoantibody bound to the patient’s RBCs (detected by the DAT),
“free” autoantibody may be detected in the plasma or serum of these Molecules lgG per red cell
patients by the IAT. In the IAT, the patient’s serum or plasma is incu-
bated with normal donor erythrocytes at the appropriate temperature
(in this case, 37°C). The cells are washed, suspended in saline solution, 1000
and then tested for agglutination by antiglobulin serum. The presence
of unbound autoantibody in plasma depends upon the total amount of
antibody being produced and the binding affinity of the antibody for Upper normal
RBC antigens. In general, patients whose RBCs are heavily coated with (40 molecules per cell)
IgG more likely exhibit plasma autoantibody. Protease-modified RBCs
are more sensitive than native RBCs in detecting plasma autoantibody, 0
but such data must be interpreted with caution, because alloantibod- 0 1+ 2+ 3+ 4+
ies, naturally occurring antibodies to cryptic antigens, and other serum Antiglobulin reaction
components may interact with enzyme-modified RBCs. Patients with Figure 54–4. Comparison of direct antiglobulin reactions (with
a positive IAT as a result of a warm-reactive autoantibody should also anti–immunoglobulin [Ig]G serum) with molecules of red blood cell–
have a positive DAT. A patient with a serum anti-RBC antibody (posi- bound IgG determined by a quantitative antibody consumption assay
tive IAT) and a negative DAT probably does not have an autoimmune (method described by Gilliland and colleagues ). The two assays were
247
process but rather an alloantibody stimulated by prior transfusion or conducted concurrently on the same blood specimen. The antiglobulin
pregnancy. reactions were performed manually and read macroscopically.
Kaushansky_chapter 54_p0823-0846.indd 834 9/19/15 12:27 AM