Page 855 - Williams Hematology ( PDFDrive )
P. 855
830 Part VI: The Erythrocyte Chapter 54: Hemolytic Anemia Resulting from Immune Injury 831
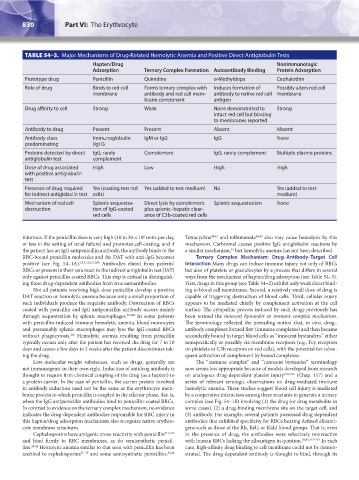
TABLE 54–3. Major Mechanisms of Drug-Related Hemolytic Anemia and Positive Direct Antiglobulin Tests
Hapten/Drug Nonimmunologic
Adsorption Ternary Complex Formation Autoantibody Binding Protein Adsorption
Prototype drug Penicillin Quinidine α-Methyldopa Cephalothin
Role of drug Binds to red cell Forms ternary complex with Induces formation of Possibly alters red cell
membrane antibody and red cell mem- antibody to native red cell membrane
brane component antigen
Drug affinity to cell Strong Weak None demonstrated to Strong
intact red cell but binding
to membranes reported
Antibody to drug Present Present Absent Absent
Antibody class Immunoglobulin IgM or IgG IgG None
predominating (Ig) G
Proteins detected by direct IgG, rarely Complement IgG, rarely complement Multiple plasma proteins
antiglobulin test complement
Dose of drug associated High Low High High
with positive antiglobulin
test
Presence of drug required Yes (coating test red Yes (added to test medium) No Yes (added to test
for indirect antiglobulin test cells) medium)
Mechanism of red cell Splenic sequestra- Direct lysis by complement Splenic sequestration None
destruction tion of IgG-coated plus splenic–hepatic clear-
red cells ance of C3b-coated red cells
injurious. If the penicillin dose is very high (10 to 30 × 10 units per day, Tetracycline 40,41 and tolbutamide 44,45 also may cause hemolysis by this
6
or less in the setting of renal failure) and promotes cell coating, and if mechanism. Carbromal causes positive IgG antiglobulin reactions by
43
the patient has an IgG antipenicillin antibody, the antibody binds to the a similar mechanism, but hemolytic anemia has not been described.
RBC-bound penicillin molecules and the DAT with anti-IgG becomes Ternary Complex Mechanism: Drug–Antibody–Target Cell
positive (see Fig. 54–1A). 29,31,32,51,208 Antibodies eluted from patients’ Interaction Many drugs can induce immune injury not only of RBCs
RBCs or present in their sera react in the indirect antiglobulin test (IAT) but also of platelets or granulocytes by a process that differs in several
only against penicillin-coated RBCs. This step is critical in distinguish- ways from the mechanism of hapten/drug adsorption (see Table 54–3).
ing these drug-dependent antibodies from true autoantibodies. First, drugs in this group (see Table 54–2) exhibit only weak direct bind-
Not all patients receiving high-dose penicillin develop a positive ing to blood cell membranes. Second, a relatively small dose of drug is
DAT reaction or hemolytic anemia because only a small proportion of capable of triggering destruction of blood cells. Third, cellular injury
such individuals produce the requisite antibody. Destruction of RBCs appears to be mediated chiefly by complement activation at the cell
coated with penicillin and IgG antipenicillin antibody occurs mainly surface. The cytopathic process induced by such drugs previously has
through sequestration by splenic macrophages. 30,209 In some patients been termed the innocent bystander or immune complex mechanism.
with penicillin-induced immune hemolytic anemia, blood monocytes The terminology reflected the prevailing notion that, in vivo, drug–
and presumably splenic macrophages may lyse the IgG-coated RBCs antibody complexes formed first (immune complexes) and then became
210
without phagocytosis. Hemolytic anemia resulting from penicillin secondarily bound to target blood cells as “innocent bystanders,” either
typically occurs only after the patient has received the drug for 7 to 10 nonspecifically or possibly via membrane receptors (e.g., Fcγ receptors
days and ceases a few days to 2 weeks after the patient discontinues tak- on platelets or C3b receptors on red cells), with the potential for subse-
ing the drug. quent activation of complement by bound complexes.
Low-molecular-weight substances, such as drugs, generally are The “immune complex” and “innocent bystander” terminology
not immunogenic in their own right. Induction of antidrug antibody is now seems less appropriate because of models developed from research
thought to require firm chemical coupling of the drug (as a hapten) to on analogous drug-dependent platelet injury 214–216 (Chap. 117) and a
a protein carrier. In the case of penicillin, the carrier protein involved series of relevant serologic observations on drug-mediated immune
in antibody induction need not be the same as the erythrocyte mem- hemolytic anemia. These studies suggest blood cell injury is mediated
brane protein to which penicillin is coupled in the effector phase, that is, by a cooperative interaction among three reactants to generate a ternary
when the IgG antipenicillin antibodies bind to penicillin-coated RBCs. complex (see Fig. 54–1B) involving (1) the drug (or drug metabolite in
In contrast to evidence on the ternary complex mechanism, no evidence some cases), (2) a drug-binding membrane site on the target cell, and
indicates the drug-dependent antibodies responsible for RBC injury in (3) antibody. For example, several patients possessed drug-dependent
this hapten/drug adsorption mechanism also recognize native erythro- antibodies that exhibited specificity for RBCs bearing defined alloanti-
cyte membrane structures. gens such as those of the Rh, Kell, or Kidd blood groups. That is, even
Cephalosporins have antigenic cross-reactivity with penicillin 211,213 in the presence of drug, the antibodies were selectively nonreactive
and bind firmly to RBC membranes, as do semisynthetic penicil- with human RBCs lacking the alloantigen in question. 58,84,217–219 In each
lins. 33,34 Hemolytic anemia similar to that seen with penicillin has been case, high-affinity drug binding to cell membrane could not be demon-
ascribed to cephalosporins 35–39 and some semisynthetic penicillins. 33,34 strated. The drug-dependent antibody is thought to bind, through its
Kaushansky_chapter 54_p0823-0846.indd 830 9/19/15 12:27 AM