Page 915 - Williams Hematology ( PDFDrive )
P. 915
890 Part VI: The Erythrocyte Chapter 58: The Porphyrias 891
1,2
Porphyrias are classified as either erythropoietic or hepatic, depend- and brown-colored bones at autopsy. In 1898, T. McCall Anderson
ing on the principal site of accumulation of pathway intermediates. described two brothers (ages 23 and 26 years) who most likely had
3
The erythropoietic porphyrias are congenital erythropoietic porphyria CEP, and suffered from hydroa aestivale, with red urine, pruritus and
(CEP), which is very rare, erythropoietic protoporphyria (EPP), the blistering of sun-exposed skin, especially in summer, leading to exten-
third most common porphyria and the most common in children, and sive scarring and mutilation of the ears and nose (see Chap. 58, Fig.
X-linked protoporphyria (XLP), which has the same phenotype as EPP 58–5). Using available methods, their urine was also demonstrated to
but is less common. Hepatic porphyrias include the acute porphyrias, contain a substance related to hematoporphyrin. In 1889, Stokvis first
4
which cause neurologic symptoms usually in the form of acute attacks, described a case of acute porphyria in an elderly woman who developed
and porphyria cutanea tarda (PCT), which is the most common of the dark-red urine and later died after taking sulphonal, a drug related to
porphyrias, and causes chronic blistering lesions on sun-exposed areas the barbiturates. 5
of the skin. The acute porphyrias include ALA dehydratase deficiency Hans Günther published a monograph on porphyrins in 1911 and
6
porphyria (ADP), acute intermittent porphyria (AIP), hereditary copro- classified porphyrias into four groups: (1) those that have an acute onset
porphyria (HCP), and variegate porphyria (VP). VP, and less commonly without association with drug ingestion, (2) those that are caused by
HCP, can also cause skin manifestations identical to those in PCT. sulphonal or trional, (3) hematoporphyria congenita, and (4) chronic
A type of porphyria is associated with loss-of-function muta- hematoporphyria. The first two groups correspond to the acute porphy-
tions of seven of the eight enzymes in the heme biosynthetic pathway rias, which may present with attacks sometimes related to ingestion of
(Table 58–1 and Fig. 58–1) PCT is primarily caused by an acquired certain drugs, the second group to CEP and hepatoerythropoietic por-
deficiency of the fifth pathway enzyme, with heterozygous mutations phyria (HEP), and the fourth to PCT. In 1923, Archibald Garrod pro-
of that enzyme contributing in some cases. Gain-of-function mutations posed the term inborn errors of metabolism for a number of inherited
of ALAS2, the erythroid form of the first pathway enzyme, cause XLP, metabolic disorders, including the porphyrias. 7
whereas loss-of-function mutations of this enzyme cause X-linked side- Sachs noted an Ehrlich-positive chromogen that was not uro-
roblastic anemia (Chap. 59). Table 58–2 summarizes the major clinical bilinogen in urine of patients with acute porphyria in 1931. In the
and laboratory features of the porphyrias. late1930s, Waldenström noted that excretion of this chromogen was an
A case of CEP reported by Schultz in 1874 was the first descrip- autosomal dominant trait in AIP families, which he identified as PBG in
8
tion of porphyria in the literature. This case was a 33-year-old man with 1939. The classification of porphyrias as erythropoietic and hepatic was
photosensitivity since age 3 months, anemia, splenomegaly, red-wine- proposed in 1954 by Schmid, Schwartz, and Watson. An epidemic of
9
colored urine as a result of a pigment resembling hematoporphyrin, hexachlorobenzene-induced PCT in eastern Turkey in 1957 10,11 provided
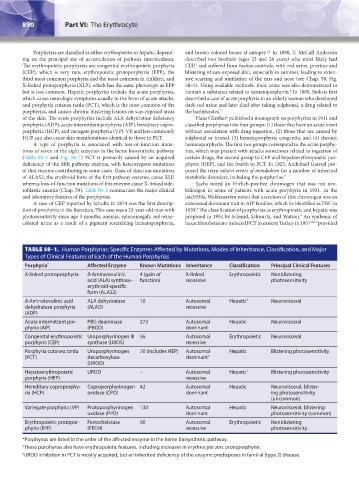
TABLE 58–1. Human Porphyrias: Specific Enzymes Affected by Mutations, Modes of Inheritance, Classification, and Major
Types of Clinical Features of Each of the Human Porphyrias
Porphyria * Affected Enzyme Known Mutations Inheritance Classification Principal Clinical Features
X-linked protoporphyria δ-Aminolevulinic 4 (gain of X-linked Erythropoietic Nonblistering
acid (ALA) synthase– function) recessive photosensitivity
erythroid-specific
form (ALAS2)
δ-Aminolevulinic acid ALA dehydratase 10 Autosomal Hepatic † Neurovisceral
dehydratase porphyria (ALAD) recessive
(ADP)
Acute intermittent por- PBG deaminase 273 Autosomal Hepatic Neurovisceral
phyria (AIP) (PBGD) dominant
Congenital erythropoietic Uroporphyrinogen III 36 Autosomal Erythropoietic Neurovisceral
porphyria (CEP) synthase (UROS) recessive
Porphyria cutanea tarda Uroporphyrinogen 70 (includes HEP) Autosomal Hepatic Blistering photosensitivity
(PCT) decarboxylase dominant ‡
(UROD)
Hepatoerythropoietic UROD – Autosomal Hepatic † Blistering photosensitivity
porphyria (HEP) recessive
Hereditary coproporphy- Coproporphyrinogen 42 Autosomal Hepatic Neurovisceral; blister-
ria (HCP) oxidase (CPO) dominant ing photosensitivity
(uncommon)
Variegate porphyria (VP) Protoporphyrinogen 130 Autosomal Hepatic Neurovisceral; blistering
oxidase (PPO) dominant photosensitivity (common)
Erythropoietic protopor- Ferrochelatase 90 Autosomal Erythropoietic Nonblistering
phyria (EPP) (FECH) recessive photosensitivity
*Porphyrias are listed in the order of the affected enzyme in the heme biosynthetic pathway.
† These porphyrias also have erythropoietic features, including increases in erythrocyte zinc protoporphyrin.
‡ UROD inhibition in PCT is mostly acquired, but an inherited deficiency of the enzyme predisposes in familial (type 2) disease.
Kaushansky_chapter 58_p0889-0914.indd 890 9/18/15 5:57 PM