Page 918 - Williams Hematology ( PDFDrive )
P. 918
892 Part VI: The Erythrocyte Chapter 58: The Porphyrias 893
Succinyl-CoA O OH a p
O OH
O OH O OH C
C CoA C C CH 2 B
2
Co 2 2, H O 4, NH 3 p N
H C CH 2 CH 2 CH 2 H a
2
H C ALAS (1) CH 2 ALAD (2) C C A NH HN C
2
C C O C CH PBGD (3) a
CoA O CH 2 H C N H p
N
H N 2 H HO D
HO C CH NH 2 2
ALA NH 2
O H PBG p a
Glycine
HMB
O
H 2
UROS (4)
a p
v
m
B
p N
B H a
N A NH HN C
m a
A N Fe N C H N p
m D
N p
D UROD (5) a p
Copro’gen III
m p m p
Uro’gen III
Heme B 4, CO 2
p N
H m
FECH (8) A NH HN C
Fe 2+ m H N p
v v D
m CPO (6)
m
B B m p
N 2, CO 2
H N
2
v m v H m 2, H O 2
A N N C
m A NH HN C
H p m
N H p
N
D PPO (7) D
m p m p
O 2
Protoporphyrin IX Proto’gen IX
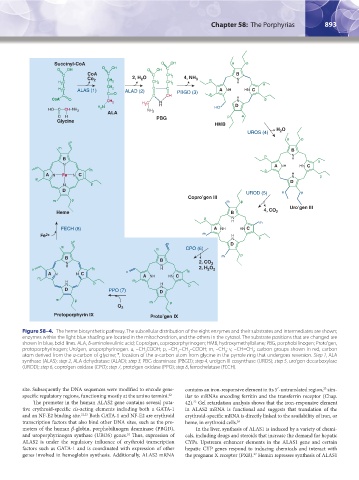
Figure 58–4. The heme biosynthetic pathway. The subcellular distribution of the eight enzymes and their substrates and intermediates are shown;
enzymes within the light blue shading are located in the mitochondrion, and the others in the cytosol. The substrate positions that are changed are
shown in blue, bold lines. ALA, δ-aminolevulinic acid; Copro’gen, coproporphyrinogen; HMB, hydroxymethylbilane; PBG, porphobilinogen; Proto’gen,
protoporphyrinogen; Uro’gen, uroporphyrinogen. a, –CH COOH; p, –CH –CH –COOH; m, –CH ; v, –CH=CH ; carbon groups shown in red, carbon
2
2
2
3
2
atom derived from the α-carbon of glycine; *, location of the α-carbon atom from glycine in the pyrrole ring that undergoes reversion. Step 1, ALA
synthase (ALAS); step 2, ALA dehydratase (ALAD); step 3, PBG deaminase (PBGD); step 4, uro’gen III cosynthase (UROS); step 5, uro’gen decarboxylase
(UROD); step 6, copro’gen oxidase (CPO); step 7, proto’gen oxidase (PPO); step 8, ferrochelatase (FECH).
site. Subsequently the DNA sequences were modified to encode gene- contains an iron-responsive element in its 5′-untranslated region, sim-
23
specific regulatory regions, functioning mostly at the amino termini. 22 ilar to mRNAs encoding ferritin and the transferrin receptor (Chap.
The promoter in the human ALAS2 gene contains several puta- 42). Gel retardation analysis shows that the iron-responsive element
25
tive erythroid-specific cis-acting elements including both a GATA-1 in ALAS2 mRNA is functional and suggests that translation of the
and an NF-E2 binding site. 22,23 Both GATA-1 and NF-E2 are erythroid erythroid-specific mRNA is directly linked to the availability of iron, or
transcription factors that also bind other DNA sites, such as the pro- heme, in erythroid cells. 26
moters of the human β-globin, porphobilinogen deaminase (PBGD), In the liver, synthesis of ALAS1 is induced by a variety of chemi-
and uroporphyrinogen synthase (UROS) genes. Thus, expression of cals, including drugs and steroids that increase the demand for hepatic
24
ALAS2 is under the regulatory influence of erythroid transcription CYPs. Upstream enhancer elements in the ALAS1 gene and certain
factors such as GATA-1 and is coordinated with expression of other hepatic CYP genes respond to inducing chemicals and interact with
genes involved in hemoglobin synthesis. Additionally, ALAS2 mRNA the pregnane X receptor (PXR). Hemin represses synthesis of ALAS1
27
Kaushansky_chapter 58_p0889-0914.indd 893 9/18/15 5:58 PM