Page 991 - Williams Hematology ( PDFDrive )
P. 991
966 Part VII: Neutrophils, Eosinophils, Basophils, and Mast Cells Chapter 63: Basophils, Mast Cells, and Related Disorders 967
DISTINGUISHING FEATURES OF precursors. 16–21 Except for a numerically minor population of mast cells
residing in the marrow (see Fig. 63–1A), this lineage completes its pro-
8
BASOPHILS AND MAST CELLS gram of maturation in other tissues. 16–21 Unlike basophils, mast cells
can be long-lived. At least some mast cells can proliferate in the tissues
BASOPHILS during a variety of inflammatory or reparative processes. 1,16,17 Studies in
Despite certain similarities in biochemistry and function, mammalian mice, rats, nonhuman primates, and humans indicate many aspects of
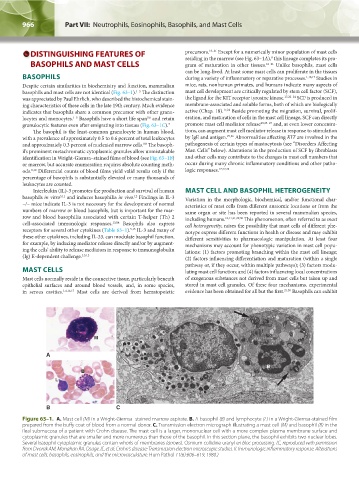
basophils and mast cells are not identical (Fig. 63–1). The distinction mast cell development are critically regulated by stem cell factor (SCF),
1–5
was appreciated by Paul Ehrlich, who described the histochemical stain- the ligand for the KIT receptor tyrosine kinase. 17,22–24 SCF is produced in
ing characteristics of these cells in the late 19th century. Much evidence membrane-associated and soluble forms, both of which are biologically
indicates that basophils share a common precursor with other granu- active (Chap. 18). 17,24 Beside promoting the migration, survival, prolif-
locytes and monocytes. Basophils have a short life span and retain eration, and maturation of cells in the mast cell lineage, SCF can directly
1–5
5,6
granulocytic features even after emigrating into tissues (Fig. 63–1C). 7 promote mast cell mediator release 23,25–27 and, at even lower concentra-
The basophil is the least-common granulocyte in human blood, tions, can augment mast cell mediator release in response to stimulation
with a prevalence of approximately 0.5 to 0.6 percent of total leukocytes by IgE and antigen. 25,26 Abnormalities affecting KIT are involved in the
and approximately 0.3 percent of nucleated marrow cells. The basoph- pathogenesis of certain types of mastocytosis (see “Disorders Affecting
8,9
il’s prominent metachromatic cytoplasmic granules allow unmistakable Mast Cells” below). Alterations in the production of SCF by fibroblasts
identification in Wright-Giemsa–stained films of blood (see Fig. 63–1B) and other cells may contribute to the changes in mast cell numbers that
or marrow, but accurate enumeration requires absolute counting meth- occur during many chronic inflammatory conditions and other patho-
ods. Differential counts of blood films yield valid results only if the logic responses. 17,22,28
9,10
percentage of basophils is substantially elevated or many thousands of
leukocytes are counted.
Interleukin (IL)-3 promotes the production and survival of human MAST CELL AND BASOPHIL HETEROGENEITY
basophils in vitro and induces basophilia in vivo. Findings in IL-3 Variation in the morphologic, biochemical, and/or functional char-
3,11
12
–/– mice indicate IL-3 is not necessary for the development of normal acteristics of mast cells from different anatomic locations or from the
numbers of marrow or blood basophils, but is important for the mar- same organ or site has been reported in several mammalian species,
row and blood basophilia associated with certain T-helper (Th) 2 including humans. 16,17,21,29,30 This phenomenon, often referred to as mast
cell-associated immunologic responses. 13,14 Basophils also express cell heterogeneity, raises the possibility that mast cells of different phe-
receptors for several other cytokines (Table 63–1). IL-3 and many of notype express different functions in health or disease and may exhibit
5,15
these other cytokines, including IL-33, can modulate basophil function, different sensitivities to pharmacologic manipulation. At least four
for example, by inducing mediator release directly and/or by augment- mechanisms may account for phenotypic variation in mast cell popu-
ing the cells’ ability to release mediators in response to immunoglobulin lations: (1) factors promoting branching within the mast cell lineage;
(Ig) E-dependent challenge. 3,5,12 (2) factors influencing differentiation and maturation (within a single
pathway or, if they occur, within multiple pathways); (3) factors modu-
MAST CELLS lating mast cell function; and (4) factors influencing local concentrations
Mast cells normally reside in the connective tissue, particularly beneath of exogenous substances not derived from mast cells but taken up and
epithelial surfaces and around blood vessels, and, in some species, stored in mast cell granules. Of these four mechanisms, experimental
in serous cavities. 1,4,16,17 Mast cells are derived from hematopoietic evidence has been obtained for all but the first. 21,30 Basophils can exhibit
M
A M
B
B
L
B C
Figure 63–1. A. Mast cell (M) in a Wright-Giemsa–stained marrow aspirate. B. A basophil (B) and lymphocyte (L) in a Wright-Giemsa-stained film
prepared from the buffy coat of blood from a normal donor. C. Transmission electron micrograph illustrating a mast cell (M) and basophil (B) in the
ileal submucosa of a patient with Crohn disease. The mast cell is a larger, mononuclear cell with a more complex plasma membrane surface and
cytoplasmic granules that are smaller and more numerous than those of the basophil. In this section plane, the basophil exhibits two nuclear lobes.
Several basophil cytoplasmic granules contain whorls of membranes (arrows). Osmium collidine uranyl en bloc processing. (C, reproduced with permission
from Dvorak AM, Monahan RA, Osage JE, et al: Crohn's disease: Transmission electron microscopic studies. II. Immunologic inflammatory response. Alterations
of mast cells, basophils, eosinophils, and the microvasculature. Hum Pathol 11(6):606–619, 1980.)
Kaushansky_chapter 63_p0965-0982.indd 966 9/18/15 11:01 PM