Page 1153 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1153
1118 Part NINE Transplantation
100 TABLE 82.2 Staging and Grading of acute
NK-ve 87%
80 Graft-Versus-Host Disease (aGvHD)
NK+ve 62% Stage Skin Liver Gastrointestinal system
(Bilirubin) (GI) (Stool output)
% surviving 0 None <2 mg/dL Adult: <500 mL/day
60
40
1 Rash <25% BSA 2–3 mg/dL Child: <10 mL/kg/day
Adult: 500–999 mL/day
20 Child: 10–19.9 mL/kg/day or
P<0.01 persistent nausea,
vomiting, or anorexia, with
0 a positive upper GI biopsy
0 2000 4000 6000 2 Rash 25–50% 3–6 mg/dL Adult: 1000–1500 mL/day
Days BSA Child: 20–30 mL/kg/day
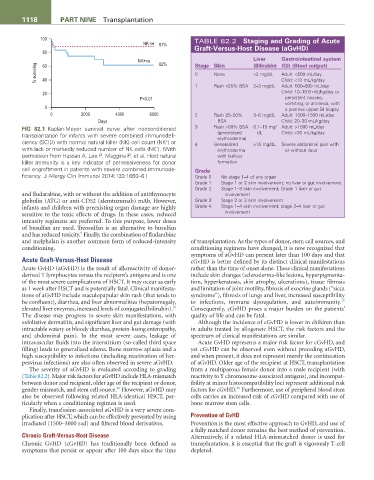
FIG 82.1 Kaplan-Meyer survival curve after nonconditioned 3 Rash >50% BSA 6.1–15 mg/ Adult: >1500 mL/day
transplantation for infants with severe combined immunodefi- (generalized dL Child: >30 mL/kg/day
erythroderma)
+
ciency (SCID) with normal natural killer (NK) cell count (NK ) or 4 Generalized >15 mg/dL Severe abdominal pain with
−
with lack or markedly reduced number of NK cells (NK ). (With erythroderma or without ileus
permission from Hassan A, Lee P, Maggina P, et al. Host natural with bullous
killer immunity is a key indicator of permissiveness for donor formation
cell engraftment in patients with severe combined immunode- Grade
ficiency. J Allergy Clin Immunol 2014;133:1660–6.) Grade 0 No stage 1–4 of any organ
Grade 1: Stage 1 or 2 skin involvement; no liver or gut involvement
Grade 2: Stage 1–3 skin involvement; Grade 1 liver or gut
and fludarabine, with or without the addition of antithymocyte involvement
globulin (ATG) or anti-CD52 (alemtuzumab) mAb. However, Grade 3: Stage 2 or 3 skin involvement
infants and children with preexisting organ damage are highly Grade 4: Stage 1–4 skin involvement; stage 2–4 liver or gut
sensitive to the toxic effects of drugs. In these cases, reduced involvement
intensity regimens are preferred. To this purpose, lower doses
of busulfan are used. Treosulfan is an alternative to busulfan
9
and has reduced toxicity. Finally, the combination of fludarabine
and melphalan is another common form of reduced-intensity of transplantation. As the types of donor, stem cell sources, and
conditioning. conditioning regimens have changed, it is now recognized that
symptoms of aGvHD can present later than 100 days and that
Acute Graft-Versus-Host Disease cGvHD is better defined by its distinct clinical manifestations
Acute GvHD (aGvHD) is the result of alloreactivity of donor- rather than the time of onset alone. These clinical manifestations
derived T lymphocytes versus the recipient’s antigens and is one include skin changes (scleroderma-like lesions, hyperpigmenta-
of the most severe complications of HSCT. It may occur as early tion, hyperkeratosis, skin atrophy, ulcerations), tissue fibrosis
as 1 week after HSCT and is potentially fatal. Clinical manifesta- and limitation of joint motility, fibrosis of exocrine glands (“sicca
tions of aGvHD include maculopapular skin rash (that tends to syndrome”), fibrosis of lungs and liver, increased susceptibility
10
be confluent), diarrhea, and liver abnormalities (hepatomegaly, to infections, immune dysregulation, and autoimmunity.
10
elevated liver enzymes, increased levels of conjugated bilirubin). Consequently, cGvHD poses a major burden on the patients’
The disease may progress to severe skin manifestations, with quality of life and can be fatal.
exfoliative dermatitis, and significant liver and gut damage (with Although the incidence of cGvHD is lower in children than
intractable watery or bloody diarrhea, protein-losing enteropathy, in adults treated by allogeneic HSCT, the risk factors and the
and abdominal pain). In the most severe cases, leakage of spectrum of clinical manifestations are similar.
intravascular fluids into the interstitium (so-called third space Acute GvHD represents a major risk factor for cGvHD, and
filling) leads to generalized edema. Bone marrow aplasia and a yet cGvHD can be observed even without preceding aGvHD,
high susceptibility to infections (including reactivation of her- and when present, it does not represent merely the continuation
pesvirus infections) are also often observed in severe aGvHD. of aGvHD. Older age of the recipient at HSCT, transplantation
The severity of aGvHD is evaluated according to grading from a multiparous female donor into a male recipient (with
(Table 82.2). Major risk factors for aGvHD include HLA-mismatch reactivity to Y chromosome-associated antigens), and incompat-
between donor and recipient, older age of the recipient or donor, ibility at minor histocompatibility loci represent additional risk
10
10
gender mismatch, and stem cell source. However, aGvHD may factors for cGvHD. Furthermore, use of peripheral blood stem
also be observed following related HLA-identical HSCT, par- cells carries an increased risk of cGvHD compared with use of
ticularly when a conditioning regimen is used. bone marrow stem cells.
Finally, transfusion-associated aGvHD is a very severe com-
plication after HSCT, which can be effectively prevented by using Prevention of GvHD
irradiated (1500–3000 rad) and filtered blood derivatives. Prevention is the most effective approach to GvHD, and use of
a fully matched donor remains the best method of prevention.
Chronic Graft-Versus-Host Disease Alternatively, if a related HLA-mismatched donor is used for
Chronic GvHD (cGvHD) has traditionally been defined as transplantation, it is essential that the graft is vigorously T-cell
symptoms that persist or appear after 100 days since the time depleted.