Page 1155 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1155
1120 Part NINE Transplantation
CLINICaL PEarLS 1.0 YEAR OF TRANSPLANTATION
Considerations of Stem Cell Transplantation
Unique to Severe Combined 0.8
Immunodeficiency (SCID)
Patients with SCID are strongly impaired in their ability to reject allogeneic 0.6 2000–2005 1995–1999
cells, including stem cells. Therefore no chemotherapy is required in
these patients in order to achieve T-cell reconstitution following stem Proportion surviving 1968–1994
cell transplantation.
The quality and the kinetics of T-cell reconstitution following stem cell 0.4
transplantation depend on the type of transplant.
If unmanipulated bone marrow from genotypically human leukocyte
antigen (HLA)-identical related donor is used, mature T cells contained 0.2 P =.0003
in the graft expand as early as 2 weeks after transplantation and
provide a rapid source of immune competence.
A similar phenomenon is also observed following unmanipulated matched 0
unrelated donor (MUD) transplantation. In this case, however, drugs
used in conditioning regimen and for prevention of graft-versus-host 0 12 24 36 60 120
disease (GvHD) decrease the degree of early expansion of donor-derived Time after transplantation (months)
T cells. Months 0 6 12 24 36 60 120
In contrast, appearance of naïve T cells occurs only at 3 months or more
after transplantation, regardless of degree of HLA matching between Number at risk
donor and recipient. Consequently, following haploidentical transplanta- 1968–1994 361 245 187 172 166 151 88
tion, there is a prolonged period during which the recipient remains 1995–1999 157 113 95 78 66 40 3
lymphopenic and at high risk of infections. 2000–2005 181 111 74 49 26 4 0
In the absence of pretransplant conditioning, following haploidentical
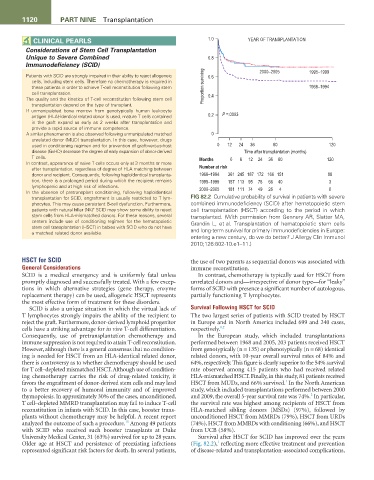
transplantation for SCID, engraftment is usually restricted to T lym- FIG 82.2 Cumulative probability of survival in patients with severe
phocytes. This may cause persistent B-cell dysfunction. Furthermore, combined immunodeficiency (SCID) after hematopoietic stem
+
patients with natural killer (NK) SCID may show some ability to reject cell transplantation (HSCT) according to the period in which
stem cells from HLA-mismatched donors. For these reasons, several transplanted. (With permission from Gennery AR, Slatter MA,
centers include use of conditioning regimen for the hematopoietic Grandin L, et al. Transplantation of hematopoietic stem cells
stem cell transplantation (HSCT) in babies with SCID who do not have and long-term survival for primary immunodeficiencies in Europe:
a matched related donor available.
entering a new century, do we do better? J Allergy Clin Immunol
2010;126:602-10.e1–11.)
HSCT for SCID the use of two parents as sequential donors was associated with
General Considerations immune reconstitution.
SCID is a medical emergency and is uniformly fatal unless In contrast, chemotherapy is typically used for HSCT from
promptly diagnosed and successfully treated. With a few excep- unrelated donors and—irrespective of donor type—for “leaky”
tions in which alternative strategies (gene therapy, enzyme forms of SCID with presence a significant number of autologous,
replacement therapy) can be used, allogeneic HSCT represents partially functioning T lymphocytes.
the most effective form of treatment for these disorders.
SCID is also a unique situation in which the virtual lack of Survival Following HSCT for SCID
T lymphocytes strongly impairs the ability of the recipient to The two largest series of patients with SCID treated by HSCT
reject the graft. Furthermore, donor-derived lymphoid progenitor in Europe and in North America included 699 and 240 cases,
cells have a striking advantage for in vivo T-cell differentiation. respectively. 1,2
Consequently, use of pretransplantation chemotherapy and In the European study, which included transplantations
immune suppression is not required to attain T-cell reconstitution. performed between 1968 and 2005, 203 patients received HSCT
However, although there is a general consensus that no condition- from genotypically (n = 135) or phenotypically (n = 68) identical
ing is needed for HSCT from an HLA-identical related donor, related donors, with 10-year overall survival rates of 84% and
there is controversy as to whether chemotherapy should be used 64%, respectively. This figure is clearly superior to the 54% survival
for T cell–depleted mismatched HSCT. Although use of condition- rate observed among 415 patients who had received related
ing chemotherapy carries the risk of drug-related toxicity, it HLA-mismatched HSCT. Finally, in this study, 81 patients received
1
favors the engraftment of donor-derived stem cells and may lead HSCT from MUDs, and 66% survived. In the North American
to a better recovery of humoral immunity and of improved study, which included transplantations performed between 2000
2
thymopoiesis. In approximately 30% of the cases, unconditioned, and 2009, the overall 5-year survival rate was 74%. In particular,
T cell–depleted MMRD transplantation may fail to induce T-cell the survival rate was highest among recipients of HSCT from
reconstitution in infants with SCID. In this case, booster trans- HLA-matched sibling donors (MSDs) (97%), followed by
plants without chemotherapy may be helpful. A recent report unconditioned HSCT from MMRDs (79%), HSCT from URDs
15
analyzed the outcome of such a procedure. Among 49 patients (74%), HSCT from MMRDs with conditioning (66%), and HSCT
with SCID who received such booster transplants at Duke from UCB (58%).
University Medical Center, 31 (63%) survived for up to 28 years. Survival after HSCT for SCID has improved over the years
1
Older age at HSCT and persistence of preexisting infections (Fig. 82.2), reflecting more effective treatment and prevention
represented significant risk factors for death. In several patients, of disease-related and transplantation-associated complications,