Page 1269 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1269
CHaPtEr 91 Immunotherapy of Allergic Disease 1229
there are group effects, there is no direct relationship to clinical factors to consider are the potential risks of emergency treat-
efficacy in individuals. ment with epinephrine and medical contraindications to VIT
(see below). Large local reactions to stings are not regarded as
SIT for Venom Anaphylaxis indications for VIT, as they are not life-threatening and do not
Anaphylaxis to Hymenoptera venom is relatively rare but can predict future problems, but there is some evidence they can be
be fatal. Venom-specific IgE antibodies can be found in 30–40% attenuated by VIT. 6
of adults for a few months following a sting, but usually these VIT accelerates the process of risk reduction and confers rapid
disappear subsequently. Some individuals develop high concentra- protection against field and laboratory stings. There is a low risk
tions of venom-specific antibodies, which may persist for many of systemic reaction (≈10%) for many years after discontinuing
years without further stings. This group of patients is at risk of VIT. Although most reactions to stings after completing VIT are
anaphylaxis to subsequent stings: a small number die of ana- mild, fatalities have been reported in patients with other risk
phylaxis each year. Fatality rates are hard to estimate, perhaps factors (mastocytosis, severe historical reactions, systemic reaction
10–20 deaths/year in the United States. during VIT). This raises the question whether VIT should be
Before embarking on venom immunotherapy (VIT), the life long, although lifelong treatment would alter the cost–benefit
patient needs to be carefully assessed, and some account should analysis. In children, the chance of systemic sting reactions remains
5
be taken of the natural history of the venom allergy. Patients <5% for up to 20 years after discontinuing VIT.
who have experienced systemic symptoms after stings are at In summary, desensitization with venom preparations provides
much greater risk of anaphylaxis on subsequent stings, compared protection against field and laboratory stings, but some patients
with patients who have only had large local reactions. The fre- will still react despite completing VIT. The residual risk of systemic
quency of systemic sting reactions in children and adults with reactions to stings after VIT is about 10%, but these are usually
a history of large local reactions is about 5–10%, whereas the mild. Patients undergoing VIT for venom anaphylaxis are usually
risk in patients with previous systemic reactions is about 30–70%. offered injectable epinephrine and other antiallergic medication
In general, there is a lower risk of repeated systemic reactions to use if stung during VIT, but this is unnecessary once they are
in children and in those with a history of milder reactions. In on maintenance doses.
adults, the risk of systemic reaction to field stings diminishes
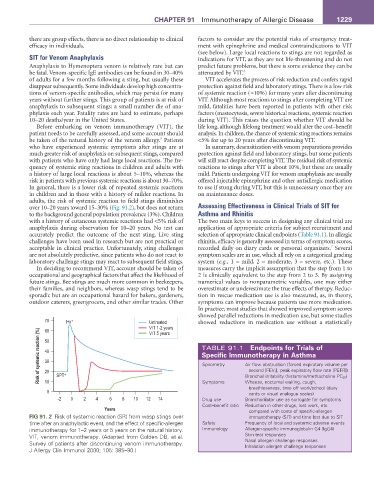
over 10–20 years toward 15–30% (Fig. 91.2), but does not return Assessing Effectiveness in Clinical Trials of SIT for
to the background general population prevalence (3%). Children Asthma and Rhinitis
with a history of cutaneous systemic reactions had <5% risk of The two main keys to success in designing any clinical trial are
anaphylaxis during observation for 10–20 years. No test can application of appropriate criteria for subject recruitment and
accurately predict the outcome of the next sting. Live sting selection of appropriate clinical endpoints (Table 91.1). In allergic
challenges have been used in research but are not practical or rhinitis, efficacy is generally assessed in terms of symptom scores,
7
acceptable in clinical practice. Unfortunately, sting challenges recorded daily on diary cards or personal organizers. Several
are not absolutely predictive, since patients who do not react to symptom scales are in use, which all rely on a categorical grading
laboratory challenge stings may react to subsequent field stings. system (e.g., 1 = mild, 2 = moderate, 3 = severe, etc.). These
In deciding to recommend VIT, account should be taken of measures carry the implicit assumption that the step from 1 to
occupational and geographical factors that affect the likelihood of 2 is clinically equivalent to the step from 2 to 3. By assigning
future stings. Bee stings are much more common in beekeepers, numerical values to nonparametric variables, one may either
their families, and neighbors, whereas wasp stings tend to be overestimate or underestimate the true effects of therapy. Reduc-
sporadic but are an occupational hazard for bakers, gardeners, tion in rescue medication use is also measured, as, in theory,
outdoor caterers, greengrocers, and other similar trades. Other symptoms can improve because patients use more medication.
In practice, most studies that showed improved symptom scores
showed parallel reductions in medication use, but some studies
70 Hx + Untreated showed reductions in medication use without a statistically
VIT 1-2 years
Risk of systemic reaction (%) 40 TABLE 91.1 Endpoints for trials of
60
VIT 5 years
50
Specific Immunotherapy in asthma
30
Spirometry
Air flow obstruction (forced expiratory volume per
second [FEV 1 ], peak expiratory flow rate [PEFR])
20
+
SPT
Bronchial irritability (histamine/methacholine PC 20 )
10
Wheeze, nocturnal waking, cough,
Symptoms
breathlessness, time off work/school (diary
0 cards or visual analogue scales)
-2 0 2 4 6 8 10 12 14 Drug use Bronchodilator use as surrogate for symptoms
Cost–benefit ratio Reduction in other drugs, lost work, etc.
Years compared with costs of specific-allergen
FIG 91. 2 Risk of systemic reaction (SR) from wasp stings over immunotherapy (SIT) and time lost due to SIT
time after an anaphylactic event, and the effect of specific-allergen Safety Frequency of local and systemic adverse events
immunotherapy for 1–2 years or 5 years on the natural history. Immunology Allergen-specific immunoglobulin G4 (IgG4)
VIT, venom immunotherapy. (Adapted from Golden DB, et al. Skin test responses
Survey of patients after discontinuing venom immunotherapy. Nasal allergen challenge responses
J Allergy Clin Immunol 2000; 105: 385–90.) Inhalation allergen challenge responses