Page 170 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 170
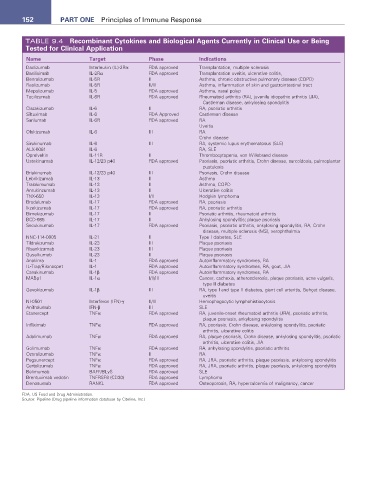
152 ParT ONE Principles of Immune Response
TABLE 9.4 recombinant Cytokines and Biological agents Currently in Clinical Use or Being
Tested for Clinical application
Name Target Phase Indications
Daclizumab Interleukin (IL)-2Rα FDA approved Transplantation, multiple sclerosis
Basiliximab IL-2Rα FDA approved Transplantation uveitis, ulcerative colitis,
Benralizumab IL-5R II Asthma, chronic obstructive pulmonary disease (COPD)
Reslizumab IL-5R II/III Asthma, inflammation of skin and gastrointestinal tract
Mepolizumab IL-5 FDA approved Asthma, nasal polyp
Tocilizumab IL-6R FDA approved Rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA),
Castleman disease, ankylosing spondylitis
Clazakizumab IL-6 II RA, psoriatic arthritis
Siltuximab IL-6 FDA Approved Castleman disease
Sarilumab IL-6R FDA approved RA
Uveitis
Olokizumab IL-6 III RA
Crohn disease
Sirukinumab IL-6 III RA, systemic lupus erythematosus (SLE)
ALX-0061 IL-6 RA, SLE
Oprelvekin IL-11R II Thrombocytopenia, von Willebrand disease
Ustekinumab IL-12/23 p40 FDA approved Psoriasis, psoriatic arthritis, Crohn disease, sarcoidosis, palmoplantar
pustulosis
Briakinumab IL-12/23 p40 III Psoriasis, Crohn disease
Lebrikizumab IL-13 II Asthma
Tralokimumab IL-13 II Asthma, COPD
Anrukinzumab IL-13 II Ulcerative colitis
TNX-650 IL-13 I/II Hodgkin lymphoma
Brodalumab IL-17 FDA approved RA, psoriasis
Ikzekizumab IL-17 FDA approved RA, psoriatic arthritis
Bimekizumab IL-17 II Psoriatic arthritis, rheumatoid arthritis
BCD-085 IL-17 II Ankylosing spondylitis; plaque psoriasis
Secukinumab IL-17 FDA approved Psoriasis, psoriatic arthritis, ankylosing spondylitis, RA, Crohn
disease, multiple sclerosis (MS), xerophthalmia
NNC-114-0005 IL-21 II Type I diabetes, SLE
Tildrakizumab IL-23 III Plaque psoriasis
Risankizumab IL-23 III Plaque psoriasis
Guselkumab IL-23 II Plaque psoriasis
Anakinra IL-1 FDA approved Autoinflammatory syndromes, RA
IL-Trap/Rilonacpet IL-1 FDA approved Autoinflammatory syndromes, RA, gout, JIA
Canakinumab IL-1β FDA approved Autoinflammatory syndromes, RA
MABp1 IL-1α I/II/III Cancer, cachexia, atherosclerosis, plaque psoriasis, acne vulgaris,
type II diabetes
Gevokizumab IL-1β III RA, type I and type II diabetes, giant cell arteritis, Behçet disease,
uveitis
NI-0501 Interferon (IFN)-γ II/III Hemophagocytic lymphohistiocytosis
Anifrolumab IFN-β III SLE
Etanercept TNFα FDA approved RA, juvenile-onset rheumatoid arthritis (JRA), psoriatic arthritis,
plaque psoriasis, ankylosing spondylitis
Infliximab TNFα FDA approved RA, psoriasis, Crohn disease, ankylosing spondylitis, psoriatic
arthritis, ulcerative colitis
Adalimumab TNFα FDA approved RA, plaque psoriasis, Crohn disease, ankylosing spondylitis, psoriatic
arthritis, ulcerative colitis, JIA
Golimumab TNFα FDA approved RA, ankylosing spondylitis, psoriatic arthritis
Ozoralizumab TNFα II RA
Pegsunercept TNFα FDA approved RA, JRA, psoriatic arthritis, plaque psoriasis, ankylosing spondylitis
Certolizumab TNFα FDA approved RA, JRA, psoriatic arthritis, plaque psoriasis, ankylosing spondylitis
Belimumab BAFF/BLyS FDA approved SLE
Brentuximab vedotin TNFRSF8 (CD30) FDA approved Lymphoma
Denosumab RANKL FDA approved Osteoporosis, RA, hypercalcemia of malignancy, cancer
FDA, US Food and Drug Administration.
Source: Pipeline (Drug pipeline information database by Citeline, Inc.)