Page 3 - PPTL.QM.001 Iss 01 - ISO 9001 Quality Manual - Full [Unsigned]
P. 3
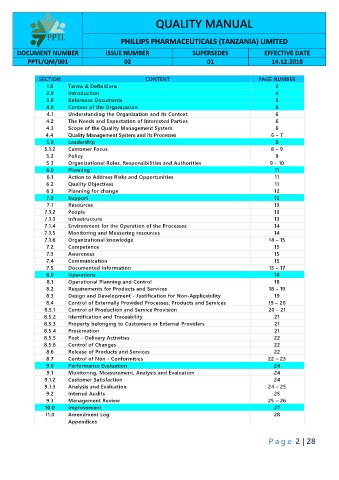
QUALITY MANUAL
PHILLIPS PHARMACEUTICALS (TANZANIA) LIMITED
DOCUMENT NUMBER ISSUE NUMBER SUPERSEDES EFFECTIVE DATE
PPTL/QM/001 02 01 14.12.2018
SECTION CONTENT PAGE NUMBER
1.0 Terms & Definitions 3
2.0 Introduction 4
3.0 Reference Documents 5
4.0 Context of the Organization 6
4.1 Understanding the Organization and its Context 6
4.2 The Needs and Expectation of Interested Parties 6
4.3 Scope of the Quality Management System 6
4.4 Quality Management System and its Processes 6 – 7
5.0 Leadership 8
5.1.2 Customer Focus 8 – 9
5.2 Policy 9
5.3 Organizational Roles, Responsibilities and Authorities 9 – 10
6.0 Planning 11
6.1 Action to Address Risks and Opportunities 11
6.2 Quality Objectives 11
6.3 Planning for change 12
7.0 Support 13
7.1 Resources 13
7.1.2 People 13
7.1.3 Infrastructure 13
7.1.4 Environment for the Operation of the Processes 14
7.1.5 Monitoring and Measuring resources 14
7.1.6 Organizational knowledge 14 – 15
7.2 Competence 15
7.3 Awareness 15
7.4 Communication 15
7.5 Documented Information 15 – 17
8.0 Operations 18
8.1 Operational Planning and Control 18
8.2 Requirements for Products and Services 18 – 19
8.3 Design and Development - Justification for Non-Applicability 19
8.4 Control of Externally Provided Processes, Products and Services 19 – 20
8.5.1 Control of Production and Service Provision 20 – 21
8.5.2 Identification and Traceability 21
8.5.3 Property belonging to Customers or External Providers 21
8.5.4 Preservation 21
8.5.5 Post – Delivery Activities 22
8.5.6 Control of Changes 22
8.6 Release of Products and Services 22
8.7 Control of Non - Conformities 22 – 23
9.0 Performance Evaluation 24
9.1 Monitoring, Measurement, Analysis and Evaluation 24
9.1.2 Customer Satisfaction 24
9.1.3 Analysis and Evaluation 24 – 25
9.2 Internal Audits 25
9.3 Management Review 25 – 26
10.0 Improvement 27
11.0 Amendment Log 28
Appendices
P a g e 2 | 28