Page 8 - PPTL.QM.001 Iss 01 - ISO 9001 Quality Manual - Full [Unsigned]
P. 8
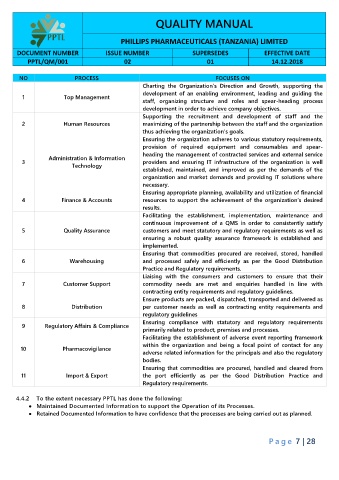
QUALITY MANUAL
PHILLIPS PHARMACEUTICALS (TANZANIA) LIMITED
DOCUMENT NUMBER ISSUE NUMBER SUPERSEDES EFFECTIVE DATE
PPTL/QM/001 02 01 14.12.2018
NO PROCESS FOCUSES ON
Charting the Organization’s Direction and Growth, supporting the
development of an enabling environment, leading and guiding the
1 Top Management
staff, organizing structure and roles and spear-heading process
development in order to achieve company objectives.
Supporting the recruitment and development of staff and the
2 Human Resources maximizing of the partnership between the staff and the organization
thus achieving the organization’s goals.
Ensuring the organization adheres to various statutory requirements,
provision of required equipment and consumables and spear-
heading the management of contracted services and external service
Administration & Information
3 providers and ensuring IT infrastructure of the organization is well
Technology
established, maintained, and improved as per the demands of the
organization and market demands and providing IT solutions where
necessary.
Ensuring appropriate planning, availability and utilization of financial
4 Finance & Accounts resources to support the achievement of the organization’s desired
results.
Facilitating the establishment, implementation, maintenance and
continuous improvement of a QMS in order to consistently satisfy
5 Quality Assurance customers and meet statutory and regulatory requirements as well as
ensuring a robust quality assurance framework is established and
implemented.
Ensuring that commodities procured are received, stored, handled
6 Warehousing and processed safely and efficiently as per the Good Distribution
Practice and Regulatory requirements.
Liaising with the consumers and customers to ensure that their
7 Customer Support commodity needs are met and enquiries handled in line with
contracting entity requirements and regulatory guidelines.
Ensure products are packed, dispatched, transported and delivered as
8 Distribution per customer needs as well as contracting entity requirements and
regulatory guidelines
Ensuring compliance with statutory and regulatory requirements
9 Regulatory Affairs & Compliance
primarily related to product, premises and processes.
Facilitating the establishment of adverse event reporting framework
within the organization and being a focal point of contact for any
10 Pharmacovigilance
adverse related information for the principals and also the regulatory
bodies.
Ensuring that commodities are procured, handled and cleared from
11 Import & Export the port efficiently as per the Good Distribution Practice and
Regulatory requirements.
4.4.2 To the extent necessary PPTL has done the following:
• Maintained Documented Information to support the Operation of its Processes.
• Retained Documented Information to have confidence that the processes are being carried out as planned.
P a g e 7 | 28