Page 7 - PRE-U STPM CHEMISTRY TERM 1
P. 7
Chemistry Term 1 STPM
2.1 Electronic Energy Levels of
Atomic Hydrogen
Electromagnetic Radiations
1 Electromagnetic radiations consist of radiations/waves such as CHAPTER
the gamma radiations, X-rays, ultraviolet radiations, infrared 2
radiations, visible light, microwaves and many others.
2 Each type of radiation is characterised by their wavelengths or
frequencies.
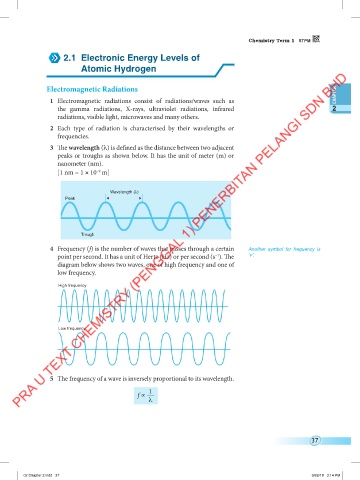
3 The wavelength (λ) is defined as the distance between two adjacent
peaks or troughs as shown below. It has the unit of meter (m) or
nanometer (nm).
[1 nm = 1 × 10 m]
–9
Wavelength (λ)
Peak
Trough
4 Frequency (f) is the number of waves that passes through a certain Another symbol for frequency is
point per second. It has a unit of Hertz (Hz) or per second (s ). The ‘v’.
–1
diagram below shows two waves, one of high frequency and one of
low frequency.
High frequency
Low frequency
5 The frequency of a wave is inversely proportional to its wavelength.
1
f ∝
λ
37
02 Chapter 2.indd 37 3/26/18 3:14 PM