Page 40 - Hybrid PBD 2022 Tg 5 - Kimia
P. 40
Kimia Tingkatan 5 Bab 3
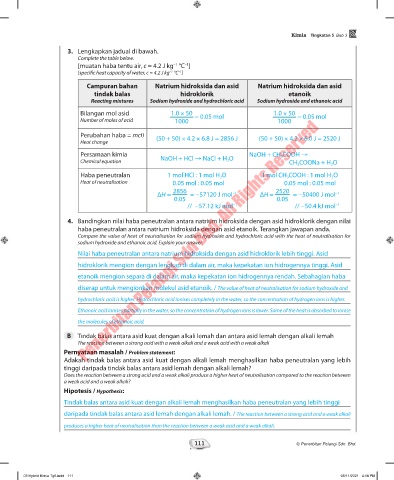
3. Lengkapkan jadual di bawah.
Complete the table below.
[muatan haba tentu air, c = 4.2 J kg °C ]
–1
–1
–1
–1
[specific heat capacity of water, c = 4.2 J kg °C ]
Campuran bahan Natrium hidroksida dan asid Natrium hidroksida dan asid
tindak balas hidroklorik etanoik
Reacting mixtures Sodium hydroxide and hydrochloric acid Sodium hydroxide and ethanoic acid
Bilangan mol asid 1.0 × 50 1.0 × 50
Penerbitan Pelangi Sdn Bhd. All Rights Reserved
Number of moles of acid 1000 = 0.05 mol 1000 = 0.05 mol
Perubahan haba = mcθ (50 + 50) × 4.2 × 6.8 J = 2856 J (50 + 50) × 4.2 × 6.0 J = 2520 J
Heat change
Persamaan kimia NaOH + CH 3 COOH →
Chemical equation NaOH + HCl → NaCl + H 2 O CH 3 COONa + H 2 O
Haba peneutralan 1 mol HCl : 1 mol H 2 O 1 mol CH 3 COOH : 1 mol H 2 O
Heat of neutralisation 0.05 mol : 0.05 mol 0.05 mol : 0.05 mol
2856 2520
ΔH = = −57120 J mol ΔH = = −50400 J mol
−1
−1
0.05 0.05
// −57.12 kJ mol −1 // −50.4 kJ mol −1
4. Bandingkan nilai haba peneutralan antara natrium hidroksida dengan asid hidroklorik dengan nilai
haba peneutralan antara natrium hidroksida dengan asid etanoik. Terangkan jawapan anda.
Compare the value of heat of neutralisation for sodium hydroxide and hydrochloric acid with the heat of neutralisation for
sodium hydroxide and ethanoic acid. Explain your answer.
Nilai haba peneutralan antara natrium hidroksida dengan asid hidroklorik lebih tinggi. Asid
hidroklorik mengion dengan lengkap di dalam air, maka kepekatan ion hidrogennya tinggi. Asid
etanoik mengion separa di dalam air, maka kepekatan ion hidrogennya rendah. Sebahagian haba
diserap untuk mengionkan molekul asid etanoik. / The value of heat of neutralisation for sodium hydroxide and
hydrochloric acid is higher. Hydrochloric acid ionises completely in the water, so the concentration of hydrogen ions is higher.
Ethanoic acid ionises partially in the water, so the concentration of hydrogen ions is lower. Some of the heat is absorbed to ionise
the molecules of ethanoic acid.
B Tindak balas antara asid kuat dengan alkali lemah dan antara asid lemah dengan alkali lemah
The reaction between a strong acid with a weak alkali and a weak acid with a weak alkali
Pernyataan masalah / Problem statement:
Adakah tindak balas antara asid kuat dengan alkali lemah menghasilkan haba peneutralan yang lebih
tinggi daripada tindak balas antara asid lemah dengan alkali lemah?
Does the reaction between a strong acid and a weak alkali produce a higher heat of neutralisation compared to the reaction between
a weak acid and a weak alkali?
Hipotesis / Hypothesis:
Tindak balas antara asid kuat dengan alkali lemah menghasilkan haba peneutralan yang lebih tinggi
daripada tindak balas antara asid lemah dengan alkali lemah. / The reaction between a strong acid and a weak alkali
produces a higher heat of neutralisation than the reaction between a weak acid and a weak alkali.
111 © Penerbitan Pelangi Sdn. Bhd.
03 Hybrid Kimia Tg5.indd 111 05/11/2021 4:18 PM