Page 44 - Hybrid PBD 2022 Tg 5 - Kimia
P. 44
Kimia Tingkatan 5 Bab 3
4. Isi pelita dengan metanol. Timbang dan rekodkan jisim pelita dan metanol, m 1 .
Fill the spirit lamp with methanol. Weigh and record the mass of the spirit lamp and methanol, m 1.
5. Letakkan pelita di bawah tin kuprum dan nyalakan sumbu pelita.
Put the spirit lamp under the copper can and light up the wick.
6. Laraskan kedudukan pelita dengan bongkah kayu. Kacau air.
Adjust the position of the lamp with the wooden block. Stir the water.
7. Padamkan sumbu pelita apabila suhu air mencapai 30 °C. Timbang dan rekodkan jisim pelita dengan
segera.
Extinguish the flame when the temperature of the water reaches 30 °C. Weigh and record the mass of the lamp immediately.
Penerbitan Pelangi Sdn Bhd. All Rights Reserved
8. Ulang langkah 1 hingga 7 dengan menggunakan etanol, propanol dan butanol.
Repeat steps 1 to 7 by using ethanol, propanol and butanol.
Keputusan/ Result:
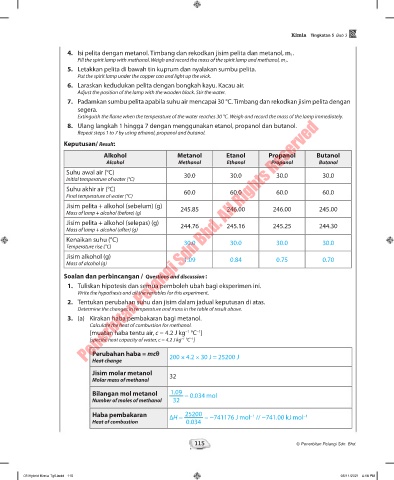
Alkohol Metanol Etanol Propanol Butanol
Alcohol Methanol Ethanol Propanol Butanol
Suhu awal air (°C) 30.0 30.0 30.0 30.0
Initial temperature of water (°C)
Suhu akhir air (°C) 60.0 60.0 60.0 60.0
Final temperature of water (°C)
Jisim pelita + alkohol (sebelum) (g) 245.85 246.00 246.00 245.00
Mass of lamp + alcohol (before) (g)
Jisim pelita + alkohol (selepas) (g) 244.76 245.16 245.25 244.30
Mass of lamp + alcohol (after) (g)
Kenaikan suhu (°C)
Temperature rise (°C) 30.0 30.0 30.0 30.0
Jisim alkohol (g) 1.09 0.84 0.75 0.70
Mass of alcohol (g)
Soalan dan perbincangan / Questions and discussion :
1. Tuliskan hipotesis dan semua pemboleh ubah bagi eksperimen ini.
Write the hypothesis and all the variables for this experiment.
2. Tentukan perubahan suhu dan jisim dalam jadual keputusan di atas.
Determine the changes in temperature and mass in the table of result above.
3. (a) Kirakan haba pembakaran bagi metanol.
Calculate the heat of combustion for methanol.
[muatan haba tentu air, c = 4.2 J kg °C ]
–1
–1
[specific heat capacity of water, c = 4.2 J kg °C ]
–1
–1
Perubahan haba = mcθ 200 × 4.2 × 30 J = 25200 J
Heat change
Jisim molar metanol 32
Molar mass of methanol
Bilangan mol metanol 1.09 = 0.034 mol
Number of moles of methanol 32
Haba pembakaran ∆H = 25200 = −741176 J mol // −741.00 kJ mol −1
−1
Heat of combustion 0.034
115 © Penerbitan Pelangi Sdn. Bhd.
03 Hybrid Kimia Tg5.indd 115 05/11/2021 4:18 PM