Page 62 - Hybrid PBD 2022 Tg 4 - Kimia
P. 62
Kimia Tingkatan 4 Bab 7
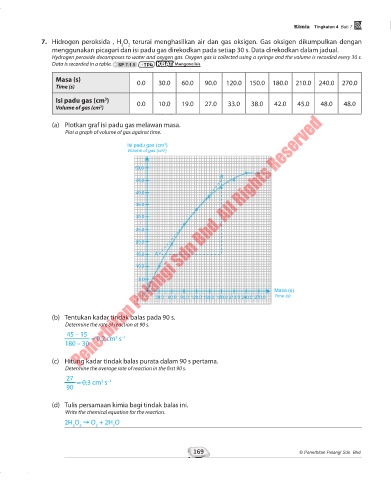
7. Hidrogen peroksida , H O terurai menghasilkan air dan gas oksigen. Gas oksigen dikumpulkan dengan
2
2
menggunakan picagari dan isi padu gas direkodkan pada setiap 30 s. Data direkodkan dalam jadual.
Hydrogen peroxide decomposes to water and oxygen gas. Oxygen gas is collected using a syringe and the volume is recorded every 30 s.
Data is recorded in a table. SP 7.1.5 TP4 KBAT Menganalisis
Masa (s) 0.0 30.0 60.0 90.0 120.0 150.0 180.0 210.0 240.0 270.0
Time (s)
Isi padu gas (cm ) 0.0 10.0 19.0 27.0 33.0 38.0 42.0 45.0 48.0 48.0
3
Volume of gas (cm )
3
Penerbitan Pelangi Sdn Bhd. All Rights Reserved
(a) Plotkan graf isi padu gas melawan masa.
Plot a graph of volume of gas against time.
Isi padu gas (cm )
3
Volume of gas (cm )
3
50.0
B
45.0
40.0
35.0
30.0
25.0
20.0
15.0 A
10.0
5.0
Masa (s)
0 30.0 60.0 90.0 120.0 150.0 180.0 210.0 240.0 270.0 Time (s)
(b) Tentukan kadar tindak balas pada 90 s.
Determine the rate of reaction at 90 s.
45 – 15
= 0.2 cm s –1
3
180 – 30
(c) Hitung kadar tindak balas purata dalam 90 s pertama.
Determine the average rate of reaction in the first 90 s.
27
= 0.3 cm s –1
3
90
(d) Tulis persamaan kimia bagi tindak balas ini.
Write the chemical equation for the reaction.
2H O ➞ O + 2H O
2
2
2
2
169 © Penerbitan Pelangi Sdn. Bhd.
07 Bab 7.indd 169 10/29/21 5:18 PM