Page 211 - ANUAL REPORT MOH 2017
P. 211
i. ’A Randomized, Double Blind, Placebo Controlled, Phase-Ii Dose Finding Study Assessing The
Safety And Efficacy Of Epicardial Injection Of Adult Human Bone Marrow Derived, Cultured,
Allogeneic Mesenchymal Stromal Cells In Patients With Ischemic Heart Disease With Low
Ejection Fraction’
ii. Phase I Clinical Study on Safety of Intravenous Allogeneic Umbilical Cord
Derived Mesenchymal Stem Cells Infusion in Healthy Volunteers
iii. ‘Transplantation of Autologous Limbal Stem Cells Via Contact Lens Delivery In Severe Ocular
Surface Disease (Multicentre Trial)
iv. Wound Healing Properties Of Local Implantation Of Autologous Peripheral Blood Mononuclear
Cells, Autologous Bone Marrow Mononuclear Cell And Allogeneic Cord-Derived Mesenchymal
Stem Cells: A Prospective, Multicentre, Randomized Trial’
v. Clinical Trial To Determine The Efficacy of Autologous Anti-Cd 19 Chimeric Antigen Receptor
T Cells (Yk-Car-T/Cd19) In Patients With Relapsed Or Refractory Cd16+ Leukemia And
Lymphoma
vi. Cytopeutics® Umbilical Cord Mesenchymal Stem Cells (Cyto-Msc) For Patients With Grade
ii-iv Acute Graft-Versus Host Disease: A Phase ii Randomized Controlled Clinical Study
vii. A Multicenter, Randomized, Open-Label, Standard Treatment-Controlled Parallel Group
Phase 2 Study To Evaluatte Efficacy And Safety On Intra-Articular Injections Of Autologous
Peripheral Blood Stem Cells And Hyaluronic Acid Adjuvant Therapy Following Subchondral
Drilling Surgery For The Treatment Of Articular Cartilage Injury In The Knee With An Optional
Open-Label Extension For The Standard Treatment-Controlled Group
viii. Production And Safety Evaluation Of GMP-Grade Human Umbilical Cord-Derived Mesenchymal
Stem Cells For Potential Clinical Applications
e. Surgical And Emergency Medicine Services Unit
Surgical Services
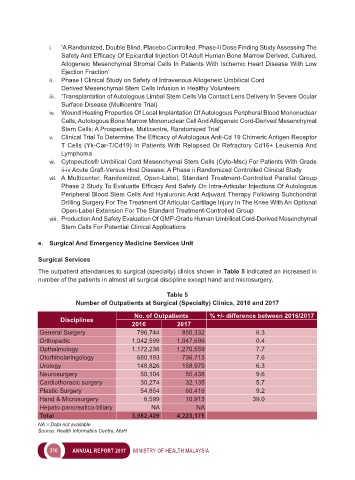
The outpatient attendances to surgical (specialty) clinics shown in Table 5 indicated an increased in
number of the patients in almost all surgical discipline except hand and microsurgery.
Table 5
Number of Outpatients at Surgical (Specialty) Clinics, 2016 and 2017
No. of Outpatients % +/- difference between 2016/2017
Disciplines
2016 2017
General Surgery 796,744 850,332 6.3
Orthopedic 1,042,599 1,047,699 0.4
Opthalmology 1,172,236 1,270,559 7.7
Otorhinolaringology 680,193 736,713 7.6
Urology 148,826 158,970 6.3
Neurosurgery 50,104 55,438 9.6
Cardiothoracic surgery 30,274 32,135 5.7
Plastic Surgery 54,854 60,416 9.2
Hand & Microsurgery 6,599 10,913 39.0
Hepato-pancreatico-biliary NA NA
Total 3,982,429 4,223,175
NA = Data not available
Source: Health Informatics Centre, MoH
210 ANNUAL REPORT 2017 MINISTRY OF HEALTH MALAYSIA