Page 416 - ANUAL REPORT MOH 2017
P. 416
PRODUCT REGISTRATION
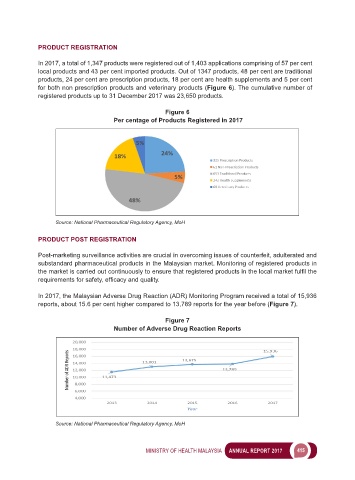
In 2017, a total of 1,347 products were registered out of 1,403 applications comprising of 57 per cent
local products and 43 per cent imported products. Out of 1347 products, 48 per cent are traditional
products, 24 per cent are prescription products, 18 per cent are health supplements and 5 per cent
for both non prescription products and veterinary products (Figure 6). The cumulative number of
registered products up to 31 December 2017 was 23,650 products.
Figure 6
Per centage of Products Registered in 2017
Source: National Pharmaceutical Regulatory Agency, MoH
PRODUCT POST REGISTRATION
Post-marketing surveillance activities are crucial in overcoming issues of counterfeit, adulterated and
substandard pharmaceutical products in the Malaysian market. Monitoring of registered products in
the market is carried out continuously to ensure that registered products in the local market fulfil the
requirements for safety, efficacy and quality.
In 2017, the Malaysian Adverse Drug Reaction (ADR) Monitoring Program received a total of 15,936
reports, about 15.6 per cent higher compared to 13,789 reports for the year before (Figure 7).
Figure 7
Number of Adverse Drug Reaction Reports
Source: National Pharmaceutical Regulatory Agency, MoH
MINISTRY OF HEALTH MALAYSIA ANNUAL REPORT 2017 415