Page 417 - ANUAL REPORT MOH 2017
P. 417
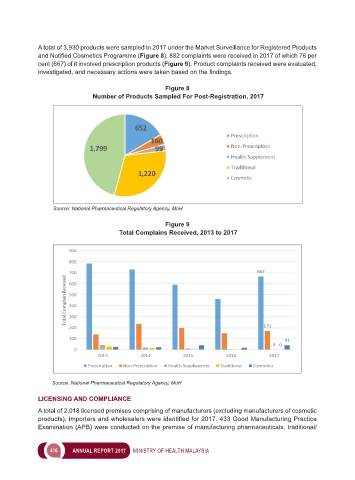
A total of 3,930 products were sampled in 2017 under the Market Surveillance for Registered Products
and Notified Cosmetics Programme (Figure 8). 882 complaints were received in 2017 of which 76 per
cent (667) of it involved prescription products (Figure 9). Product complaints received were evaluated,
investigated, and necessary actions were taken based on the findings.
Figure 8
Number of Products Sampled For Post-Registration, 2017
Source: National Pharmaceutical Regulatory Agency, MoH
Figure 9
Total Complains Received, 2013 to 2017
Source: National Pharmaceutical Regulatory Agency, MoH
LICENSING AND COMPLIANCE
A total of 2,018 licensed premises comprising of manufacturers (excluding manufacturers of cosmetic
products), importers and wholesalers were identified for 2017. 433 Good Manufacturing Practice
Examination (APB) were conducted on the premise of manufacturing pharmaceuticals, traditional/
416 ANNUAL REPORT 2017 MINISTRY OF HEALTH MALAYSIA