Page 420 - ANUAL REPORT MOH 2017
P. 420
DIRECTOR GENERAL OF HEALTH(DG)/SENIOR DIRECTOR OF PHARMACEUTICAL SERVICES
(SDPS) SPECIAL APPROVAL DRUGS
Special approval from DG/SDPS is required to obtain and use drugs outside the MOHMF. It can be
used as a last resort of treatment after all options available in the MOHMF has been exhausted. In
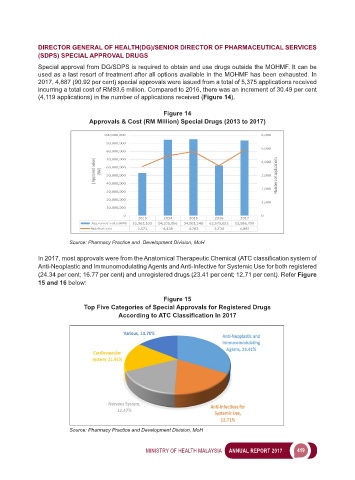
2017, 4,887 (90.92 per cent) special approvals were issued from a total of 5,375 applications received
incurring a total cost of RM93.6 million. Compared to 2016, there was an increment of 30.49 per cent
(4,119 applications) in the number of applications received (Figure 14).
Figure 14
Approvals & Cost (RM Million) Special Drugs (2013 to 2017)
Source: Pharmacy Practice and Development Division, MoH
In 2017, most approvals were from the Anatomical Therapeutic Chemical (ATC classification system of
Anti-Neoplastic and Immunomodulating Agents and Anti-Infective for Systemic Use for both registered
(24.34 per cent; 16.77 per cent) and unregistered drugs (23.41 per cent; 12.71 per cent). Refer Figure
15 and 16 below:
Figure 15
Top Five Categories of Special Approvals for Registered Drugs
According to ATC Classification In 2017
Source: Pharmacy Practice and Development Division, MoH
MINISTRY OF HEALTH MALAYSIA ANNUAL REPORT 2017 419