Page 27 - Nilam_Publication_module_Chemistry_Form.pdf
P. 27
Chemistry Form 4 • MODULE
MOLE CONCEPT / KONSEP MOL
Mole and the Number of Particles / Bilangan Mol dan Bilangan Zarah
1 To describe the amount of atoms, ions or molecules, mole is used.
Untuk menyatakan jumlah atom, ion atau molekul, unit mol digunakan.
2 A mole is an amount of substance that contains as many particles as the number of atoms in exactly 12 g of carbon-12.
Satu mol ialah jumlah bahan yang mengandungi bilangan zarah seperti mana yang terdapat dalam 12 g atom karbon-12.
3 A mole of a substance is the amount of substance which contains a constant number of particles (atoms, ions,
molecules), which is 6.02 × 10 .
23
Satu mol bahan adalah jumlah bahan yang mengandungi bilangan zarah yang tetap (atom, molekul, ion) iaitu 6.02 × 10 .
23
4 The number 6.02 × 10 is called the Avogadro Constant or Avogadro Number (N ).
23
A
Nombor 6.02 × 10 dikenali sebagai Pemalar Avogadro atau Nombor Avogadro (N ).
23
A
5 For compounds that exist as molecules/ions, the number of atoms/ions in that compound must be known.
Bagi sebatian yang wujud dalam bentuk molekul/ion, bilangan atom/ion dalam sebatian itu mestilah diketahui.
6 The symbol of mole is mol.
Simbol untuk mol ialah mol.
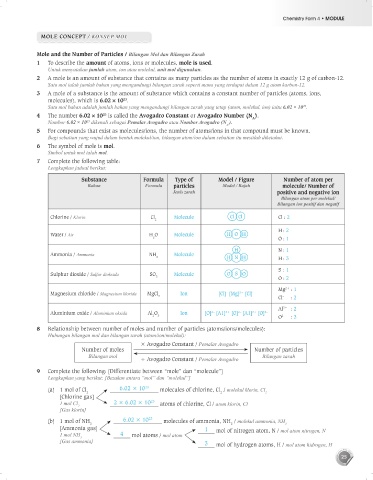
7 Complete the following table:
Lengkapkan jadual berikut:
Substance Formula Type of Model / Figure Number of atom per
Bahan Formula particles Model / Rajah molecule/ Number of
Jenis zarah positive and negative ion
Bilangan atom per molekul/
Bilangan ion positif dan negatif
Chlorine / Klorin Cl Molecule Cl Cl Cl : 2
2
H : 2
Water / Air H O Molecule H O H
2 O : 1
H N : 1
Ammonia / Ammonia NH Molecule
3 H N H H : 3
S : 1
Sulphur dioxide / Sulfur dioksida SO Molecule O S O
2 O : 2
Mg : 1
2+
–
2+
Magnesium chloride / Magnesium klorida MgCl Ion [Cl] [Mg] [Cl] –
2 Cl : 2
–
Al : 2
3+
3+
3+
2–
2–
Aluminium oxide / Aluminium oksida Al O Ion [O] [A1] [O] [A1] [O] 2–
2 3 O : 3
2–
8 Relationship between number of moles and number of particles (atoms/ions/molecules):
Hubungan bilangan mol dan bilangan zarah (atom/ion/molekul):
× Avogadro Constant / Pemalar Avogadro
Number of moles Number of particles
Bilangan mol Bilangan zarah
÷ Avogadro Constant / Pemalar Avogadro
9 Complete the following: [Differentiate between “mole” dan “molecule”]
Lengkapkan yang berikut: [Bezakan antara “mol” dan “molekul”]
(a) 1 mol of Cl 2 6.02 × 10 23 molecules of chlorine, Cl / molekul klorin, Cl 2
2
[Chlorine gas]
1 mol Cl 2 2 × 6.02 × 10 23 atoms of chlorine, Cl / atom klorin, Cl
[Gas klorin]
(b) 1 mol of NH 3 6.02 × 10 23 molecules of ammonia, NH / molekul ammonia, NH 3
3
[Ammonia gas] 1 mol of nitrogen atom, N / mol atom nitrogen, N
1 mol NH 3 4 mol atoms / mol atom
[Gas ammonia]
3 mol of hydrogen atoms, H / mol atom hidrogen, H
25
Nilam Publication Sdn. Bhd.
02-Chem F4 (3P).indd 25 12/9/2011 5:59:05 PM