Page 30 - Nilam_Publication_module_Chemistry_Form.pdf
P. 30
MODULE • Chemistry Form 4
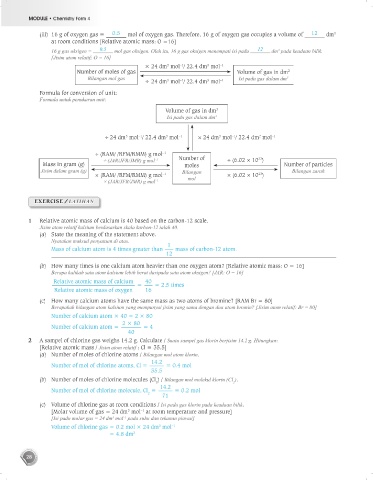
(iii) 16 g of oxygen gas = 0.5 mol of oxygen gas. Therefore, 16 g of oxygen gas occupies a volume of 12 dm
3
at room conditions [Relative atomic mass: O =16]
3
16 g gas oksigen = 0.5 mol gas oksigen. Oleh itu, 16 g gas oksigen menempati isi padu 12 dm pada keadaan bilik.
[Jisim atom relatif; O = 16]
× 24 dm mol / 22.4 dm mol –1
–1
3
3
Number of moles of gas Volume of gas in dm 2
Bilangan mol gas Isi padu gas dalam dm 3
÷ 24 dm mol / 22.4 dm mol –1
–1
3
3
Formula for conversion of unit:
Formula untuk penukaran unit:
Volume of gas in dm 3
Isi padu gas dalam dm 3
–1
÷ 24 dm mol / 22.4 dm mol –1 × 24 dm mol / 22.4 dm mol –1
3
3
3
–1
3
÷ (RAM/ /RFM/RMM) g mol –1 Number of 23
Mass in gram (g) ÷ (JAR/JFR/JMR) g mol –1 moles ÷ (6.02 × 10 ) Number of particles
Jisim dalam gram (g) Bilangan Bilangan zarah
× (RAM/ /RFM/RMM) g mol –1 × (6.02 × 10 )
23
× (JAR/JFR/JMR) g mol –1 mol
EXERCISE / LATIHAN
1 Relative atomic mass of calcium is 40 based on the carbon-12 scale.
Jisim atom relatif kalsium berdasarkan skala karbon-12 ialah 40.
(a) State the meaning of the statement above.
Nyatakan maksud penyataan di atas. 1
Mass of calcium atom is 4 times greater than mass of carbon-12 atom.
12
(b) How many times is one calcium atom heavier than one oxygen atom? [Relative atomic mass: O = 16]
Berapa kalikah satu atom kalsium lebih berat daripada satu atom oksigen? [JAR: O = 16]
Relative atomic mass of calcium 40
= = 2.5 times
Relative atomic mass of oxygen 16
(c) How many calcium atoms have the same mass as two atoms of bromine? [RAM Br = 80]
Berapakah bilangan atom kalsium yang mempunyai jisim yang sama dengan dua atom bromin? [Jisim atom relatif: Br = 80]
Number of calcium atom × 40 = 2 × 80
2 × 80
Number of calcium atom = = 4
40
2 A sampel of chlorine gas weighs 14.2 g. Calculate / Suatu sampel gas klorin berjisim 14.2 g. Hitungkan:
[Relative atomic mass / Jisim atom relatif : Cl = 35.5]
(a) Number of moles of chlorine atoms / Bilangan mol atom klorin.
14.2
Number of mol of chlorine atoms, Cl = = 0.4 mol
35.5
(b) Number of moles of chlorine molecules (Cl ) / Bilangan mol molekul klorin (Cl ).
2 2
Number of mol of chlorine molecule, Cl = 14.2 = 0.2 mol
2
71
(c) Volume of chlorine gas at room conditions / Isi padu gas klorin pada keadaan bilik.
[Molar volume of gas = 24 dm mol at room temperature and pressure]
–1
3
–1
3
[Isi padu molar gas = 24 dm mol pada suhu dan tekanan piawai]
Volume of chlorine gas = 0.2 mol × 24 dm mol
–1
3
= 4.8 dm 3
28
Nilam Publication Sdn. Bhd.
02-Chem F4 (3P).indd 28 12/9/2011 5:59:06 PM