Page 29 - Nilam_Publication_module_Chemistry_Form.pdf
P. 29
Chemistry Form 4 • MODULE
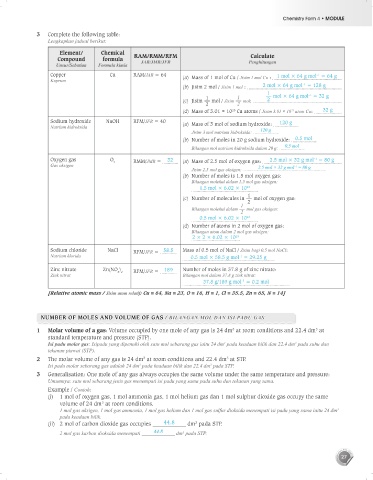
3 Complete the following table:
Lengkapkan jadual berikut:
Element/ Chemical RAM/RMM/RFM Calculate
Compound formula
Unsur/Sebatian Formula kimia JAR/JMR/JFR Penghitungan
Copper Cu RAM/JAR = 64 (a) Mass of 1 mol of Cu / Jisim 1 mol Cu : 1 mol × 64 g mol = 64 g
–1
Kuprum
–1
(b) Jisim 2 mol / Jisim 1 mol : 2 mol × 64 g mol = 128 g
1
–1
1 1 mol × 64 g mol = 32 g
(c) Jisim mol / Jisim mol: 2
2 2
(d) Mass of 3.01 × 10 Cu atoms / Jisim 3.01 × 10 atom Cu: 32 g
23
23
Sodium hydroxide NaOH RFM/JFR = 40 (a) Mass of 3 mol of sodium hydroxide: 120 g
Natrium hidroksida
Jisim 3 mol natrium hidroksida: 120 g
(b) Number of moles in 20 g sodium hydroxide: 0.5 mol
0.5 mol
Bilangan mol natrium hidroksida dalam 20 g:
Oxygen gas O 2 RMM/JMR = 32 (a) Mass of 2.5 mol of oxygen gas: 2.5 mol × 32 g mol = 80 g
–1
Gas oksigen –1
Jisim 2.5 mol gas oksigen: 2.5 mol × 32 g mol = 80 g
(b) Number of moles is 1.5 mol oxygen gas:
Bilangan molekul dalam 1.5 mol gas oksigen:
1.5 mol × 6.02 × 10 23
1
(c) Number of molecules in 2 mol of oxygen gas:
1
Bilangan molekul dalam mol gas oksigen:
2
0.5 mol × 6.02 × 10 23
(d) Number of atoms in 2 mol of oxygen gas:
Bilangan atom dalam 2 mol gas oksigen:
2 × 2 × 6.02 × 10 23
Sodium chloride NaCl RFM/JFR = 58.5 Mass of 0.5 mol of NaCl / Jisim bagi 0.5 mol NaCl:
Natrium klorida 0.5 mol × 58.5 g mol = 29.25 g
–1
Zinc nitrate Zn(NO ) RFM/JFR = 189 Number of moles in 37.8 g of zinc nitrate:
3 2
Zink nitrat Bilangan mol dalam 37.8 g zink nitrat:
37.8 g/189 g mol = 0.2 mol
–1
[Relative atomic mass / Jisim atom relatif: Cu = 64, Na = 23, O = 16, H = 1, Cl = 35.5, Zn = 65, N = 14]
NUMBER OF MOLES AND VOLUME OF GAS / BILANGAN MOL DAN ISI PADU GAS
3
1 Molar volume of a gas: Volume occupied by one mole of any gas is 24 dm at room conditions and 22.4 dm at
3
standard temperature and pressure (STP).
3
3
Isi padu molar gas: Isipadu yang dipenuhi oleh satu mol sebarang gas iaitu 24 dm pada keadaan bilik dan 22.4 dm pada suhu dan
tekanan piawai (STP).
2 The molar volume of any gas is 24 dm at room conditions and 22.4 dm at STP.
3
3
Isi padu molar sebarang gas adalah 24 dm pada keadaan bilik dan 22.4 dm pada STP.
3
3
3 Generalisation: One mole of any gas always occupies the same volume under the same temperature and pressure:
Umumnya: satu mol sebarang jenis gas menempati isi padu yang sama pada suhu dan tekanan yang sama.
Example / Contoh:
(i) 1 mol of oxygen gas, 1 mol ammonia gas, 1 mol helium gas dan 1 mol sulphur dioxide gas occupy the same
volume of 24 dm at room conditions.
3
1 mol gas oksigen, 1 mol gas ammonia, 1 mol gas helium dan 1 mol gas sulfur dioksida menempati isi padu yang sama iaitu 24 dm
3
pada keadaan bilik.
(ii) 2 mol of carbon dioxide gas occupies 44.8 dm pada STP.
3
2 mol gas karbon dioksida menempati 44.8 dm pada STP.
3
27
Nilam Publication Sdn. Bhd.
02-Chem F4 (3P).indd 27 12/9/2011 5:59:06 PM