Page 39 - Nilam_Publication_module_Chemistry_Form.pdf
P. 39
Chemistry Form 4 • MODULE
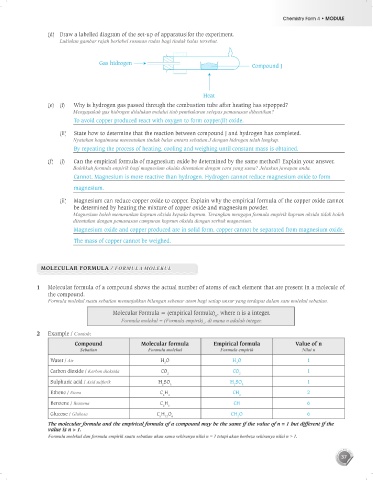
(d) Draw a labelled diagram of the set-up of apparatus for the experiment.
Lukiskan gambar rajah berlabel susunan radas bagi tindak balas tersebut.
Gas hidrogen
Compound J
Heat
(e) (i) Why is hydrogen gas passed through the combustion tube after heating has stpopped?
Mengapakah gas hidrogen dilalukan melalui tiub pembakaran selepas pemanasan dihentikan?
To avoid copper produced react with oxygen to form copper(II) oxide.
(ii) State how to determine that the reaction between compound J and hydrogen has completed.
Nyatakan bagaimana menentukan tindak balas antara sebatian J dengan hidrogen telah lengkap.
By repeating the process of heating, cooling and weighing until constant mass is obtained.
(f) (i) Can the empirical formula of magnesium oxide be determined by the same method? Explain your answer.
Bolehkah formula empirik bagi magnesium oksida ditentukan dengan cara yang sama? Jelaskan jawapan anda.
Cannot. Magnesium is more reactive than hydrogen. Hydrogen cannot reduce magnesium oxide to form
magnesium.
(ii) Magnesium can reduce copper oxide to copper. Explain why the empirical formula of the copper oxide cannot
be determined by heating the mixture of copper oxide and magnesium powder.
Magnesium boleh menurunkan kuprum oksida kepada kuprum. Terangkan mengapa formula empirik kuprum oksida tidak boleh
ditentukan dengan pemanasan campuran kuprum oksida dengan serbuk magnesium.
Magnesium oxide and copper produced are in solid form, copper cannot be separated from magnesium oxide.
The mass of copper cannot be weighed.
MOLECULAR FORMULA / FORMULA MOLEKUL
1 Molecular formula of a compound shows the actual number of atoms of each element that are present in a molecule of
the compound.
Formula molekul suatu sebatian menunjukkan bilangan sebenar atom bagi setiap unsur yang terdapat dalam satu molekul sebatian.
Molecular Formula = (empirical formula) , where n is a integer.
n
Formula molekul = (Formula empirik) , di mana n adalah integer.
n
2 Example / Contoh:
Compound Molecular formula Empirical formula Value of n
Sebatian Formula molekul Formula empirik Nilai n
Water / Air H O H O 1
2 2
Carbon dioxide / Karbon dioksida CO CO 1
2 2
Sulphuric acid / Asid sulfurik H SO H SO 1
2 4 2 4
Ethene / Etena C H CH 2
2 4 2
Benzene / Benzena C H CH 6
6 6
Glucose / Glukosa C H O CH O 6
6 12 6 2
The molecular formula and the empirical formula of a compound may be the same if the value of n = 1 but different if the
value is n > 1.
Formula molekul dan formula empirik suatu sebatian akan sama sekiranya nilai n = 1 tetapi akan berbeza sekiranya nilai n > 1.
37
Nilam Publication Sdn. Bhd.
02-Chem F4 (3P).indd 37 12/9/2011 5:59:08 PM