Page 42 - Nilam_Publication_module_Chemistry_Form.pdf
P. 42
MODULE • Chemistry Form 4
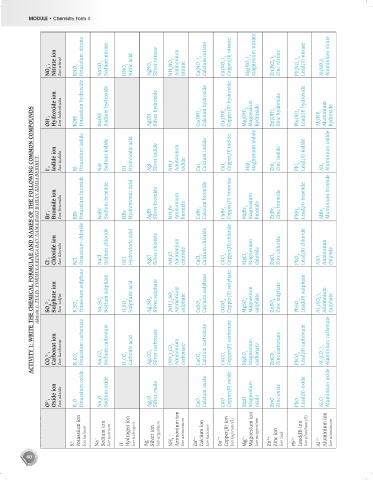
– , Nitrate ion Ion nitrat Potassium nitrate Sodium nitrate Nitric acid Silver nitrate NH 4 NO 3 Ammonium Ca(NO 3 ) 2 Calcium nitrate Cu(NO 3 ) 2 Copper(II) nitrate Mg(NO 3 ) 2 Magnesium nitrate Zn(NO 3 ) 2 Zinc nitrate Pb(NO 3 ) 2 Lead(II) nitrate Al(NO 3 ) 3 Aluminium nirate
NO 3 KNO 3 NaNO 3 HNO 3 AgNO 3 nitrate
Hydroxide ion Ion hidroksida Potassium hydroxide Sodium hydroxide Silver hydroxide Calcium hydroxide Copper(II) hydroxide Magnesium Zinc hydroxide Lead(II) hydroxide Aluminium
ACTIVITY 1: WRITE THE CHEMICAL FORMULAE AND NAMES OF THE FOLLOWING COMMON COMPOUNDS
OH – , KOH NaOH AgOH Ca(OH) 2 Cu(OH) 2 Mg(OH) 2 hydroxide Zn(OH) 2 Pb(OH) 2 Al(OH) 3 hydroxide
Iodide ion Ion iodida Potassium iodide Sodium iodide Hydroiodic acid Silver iodide Ammonium Calcium iodide Copper(II) iodide Magnesium iodide Zinc iodide Lead(II) iodide Aluminium iodide
Aktiviti 1: TULIS FORMULA KIMIA DAN NAMA BAGI BAHAN KIMIA BERIKUT
I – , KI NaI HI AgI NH 4 I iodide CaI 2 CuI 2 MgI 2 ZnI 2 PbI 2 AlI 3
Bromide ion Ion bromida Potassium bromide Sodium bromide Hydrobromic acid Silver bromide Ammonium Calcium bromide Copper(II) bromide Magnesium Zinc bromide Lead(II) bromide Aluminium bromide
Br – , KBr NaBr HBr AgBr NH 4 Br bromide CaBr 2 CuBr 2 MgBr 2 bromide ZnBr 2 PbBr 2 AlBr 3
Chloride ion Ion klorida Potassium chloride Sodium chloride Hydrocloric acid Silver chloride Ammonium Calcium chloride Copper(II) chloride Magnesium Zinc chloride Lead(II) chloride Aluminium
Cl – , KCl NaCl HCl AgCl NH 4 Cl chloride CaCl 2 CuCl 2 MgCl 2 chloride ZnCl 2 PbCl 2 AlCl 3 chloride
Sulphate ion Potassium sulphate Sodium sulphate Sulphuric acid Silver sulphate Calcium sulphate Copper(II) sulphate Zinc sulphate Lead(II) sulphate
2– , Ion sulfat (NH 4 ) 2 SO 4 Ammonium sulphate Magnesium sulphate Al 2 (SO 4 ) 3 Aluminium sulphate
SO 4 K 2 SO 4 Na 2 SO 4 H 2 SO 4 Ag 2 SO 4 CaSO 4 CuSO 4 MgSO 4 ZnSO 4 PbSO 4
Carbonat ion Potassium carbonate Sodium carbonate Carbonic acid Silver carbonate Calcium carbonate Copper(II) carbonate Zinc carbonate Lead(II) carbonate Aluminium carbonate
2– , Ion karbonat (NH 4 ) 2 CO 3 Ammonium carbonate Magnesium carbonate Al 2 (CO 3 ) 3
CO 3 K 2 CO 3 Na 2 CO 3 H 2 CO 3 Ag 2 CO 3 CaCO 3 CuCO 3 MgCO 3 ZnCO 3 PbCO 3
Oxide ion Ion oksida Potassium oxide Sodium oxide Silver oxide Calcium oxide Copper(II) oxide Magnesium Zinc oxide Lead(II) oxide Aluminium oxide
O 2– , K 2 O Na 2 O Ag 2 O CaO CuO MgO oxide ZnO PbO Al 2 O 3
Potassium ion Ion kalium Sodium ion Ion natrium Hydrogen ion Ion hidrogen Silver ion Ion argentum + Ammonium ion Ion ammonium Calcium ion Ion kalsium Copper(II) ion Ion kuprum(II) Magnesium ion Ion magnesium Lead(II) ion Ion plumbum(II) Aluminium ion Ion aluminium
K + Na + H + Ag + NH 4 Ca 2+ Cu 2+ Mg 2+ Zn 2+ Zinc ion Ion zink Pb 2+ Al 3+
40
Nilam Publication Sdn. Bhd.
02-Chem F4 (3P).indd 40 12/9/2011 5:59:08 PM