Page 43 - Nilam_Publication_module_Chemistry_Form.pdf
P. 43
Chemistry Form 4 • MODULE
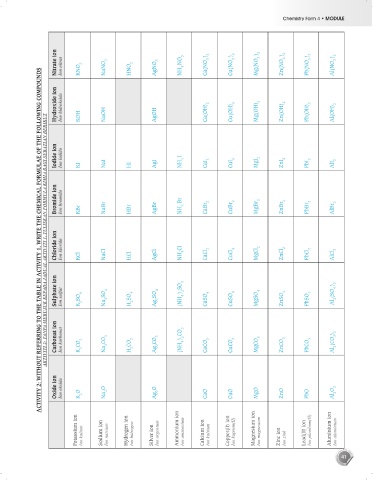
Nitrate ion Ion nitrat KNO 3 NaNO 3 HNO 3 AgNO 3 NH 4 NO 3 Ca(NO 3 ) 2 Cu(NO 3 ) 2 Mg(NO 3 ) 2 Zn(NO 3 ) 2 Pb(NO 3 ) 2 Al(NO 3 ) 3
ACTIVITY 2: WITHOUT REFERRING TO THE TABLE IN ACTIVITY 1, WRITE THE CHEMICAL FORMULAE OF THE FOLLOWING COMPOUNDS
Hydroxide ion Ion hidroksida KOH NaOH AgOH Ca(OH) 2 Cu(OH) 2 Mg(OH) 2 Zn(OH) 2 Pb(OH) 2 Al(OH) 3
AKTIVITI 2: TANPA MERUJUK KEPADA JADUAL AKTIVITI 1, TULISKAN FORMULA KIMIA BAGI SEBATIAN BERIKUT
Iodide ion Ion iodida KI NaI HI AgI NH 4 I CaI 2 CuI 2 MgI 2 ZnI 2 PbI 2 AlI 3
Bromide ion Ion bromida KBr NaBr HBr AgBr NH 4 Br CaBr 2 CuBr 2 MgBr 2 ZnBr 2 PbBr 2 AlBr 3
Chloride ion Ion klorida KCl NaCl HCl AgCl NH 4 Cl CaCl 2 CuCl 2 MgCl 2 ZnCl 2 PbCl 2 AlCl 3
Sulphate ion Ion sulfat K 2 SO 4 Na 2 SO 4 H 2 SO 4 Ag 2 SO 4 (NH 4 ) 2 SO 4 CaSO 4 CuSO 4 MgSO 4 ZnSO 4 PbSO 4 Al 2 (SO 4 ) 3
Carbonat ion Ion karbonat K 2 CO 3 Na 2 CO 3 H 2 CO 3 Ag 2 CO 3 (NH 4 ) 2 CO 3 CaCO 3 CuCO 3 MgCO 3 ZnCO 3 PbCO 3 Al 2 (CO 3 ) 3
Oxide ion Ion oksida K 2 O Na 2 O Ag 2 O CaO CuO MgO ZnO PbO Al 2 O 3
Potassium ion Ion kalium Sodium ion Ion natrium Hydrogen ion Ion hidrogen Silver ion Ion argentum Ammonium ion Ion ammonium Calcium ion Ion kalsium Copper(II) ion Ion kuprum(II) Magnesium ion Ion magnesium Zinc ion Ion zink Lead(II) ion Ion plumbum(II) Aluminium ion Ion aluminium
41
Nilam Publication Sdn. Bhd.
02-Chem F4 (3P).indd 41 12/9/2011 5:59:09 PM