Page 65 - Nilam_Publication_module_Chemistry_Form.pdf
P. 65
Chemistry Form 4 • MODULE
Experiment (a), (b) dan (c) show that all halogens have similar chemical properties but their reactivity
decreases going down the group:
Eksperimen (a), (b) dan (c) menunjukkan semua halogen menunjukkan sifat kimia yang sama tetapi kereaktifannya
berkurang apabila menuruni kumpulan.
Reactivity decreases / Kereaktifan berkurang
F , Cl , Br and I / F , Cl , Br dan I
2 2 2 2 2 2 2 2
PERIOD / KALA
1 Horizontal rows in the periodic table / Baris mendatar dalam Jadual Berkala.
2 There are seven periods known as Period 1, 2, 3, 4, 5, 6, 7 / Terdapat 7 kala ditulis sebagai Kala 1, 2, 3, 4, 5, 6, 7.
3 The number of period of an element represents the number of shells occupy with electrons in each atom of element.
Nombor kala suatu unsur mewakili bilangan petala yang diisi oleh elektron di dalam setiap atom unsur.
Elements Proton number Electron arrangement Number of shells Period
Unsur Nombor proton Susunan elektron Bilangan petala Kala
Li 3 2.1 2 2
Na 11 2.8.1 3 3
K 19 2.8.8.1 4 4
4 Period 3 elements (complete the following table): / Unsur Kala 3 (lengkapkan jadual berikut):
Elements / Unsur Na Mg Al Si P S Cl Ar
Proton number 11 12 13 14 15 16 17 18
Nombor proton
Electron arrangement 2.8.1 2.8.2 2.8.3 2.8.4 2.8.5 2.8.6 2.8.7 2.8.8
Susunan elektron
Number of shells 3 3 3 3 3 3 3 3
Bilangan petala
Positive charge in the nucleus +11 +12 +13 +14 +15 +16 +17 +18
Bilangan cas positif dalam nukleus
Radius (nm) 0.191 0.160 0.130 0.118 0.110 0.102 0.099 0.095
Jejari (nm)
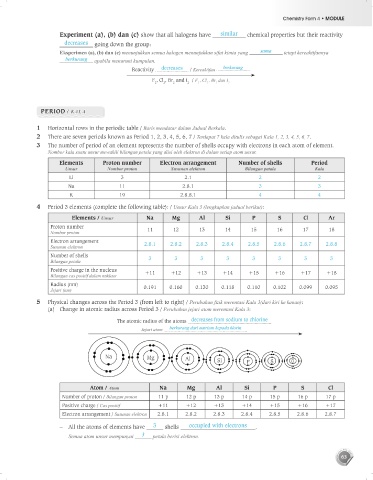
5 Physical changes across the Period 3 (from left to right) / Perubahan fizik merentasi Kala 3(dari kiri ke kanan):
(a) Change in atomic radius across Period 3 / Perubahan jejari atom merentasi Kala 3:
The atomic radius of the atoms decreases from sodium to chlorine
Jejari atom berkurang dari natrium kepada klorin
Na Mg Al Si P S Cl
16 p
Atom / Atom Na Mg Al Si P S Cl
Number of proton / Bilangan proton 11 p 12 p 13 p 14 p 15 p 16 p 17 p
Positive charge / Cas positif +11 +12 +13 +14 +15 +16 +17
Electron arrangement / Susunan elektron 2.8.1 2.8.2 2.8.3 2.8.4 2.8.5 2.8.6 2.8.7
– All the atoms of elements have 3 shells occupied with electrons .
Semua atom unsur mempunyai 3 petala berisi elektron.
63
Nilam Publication Sdn. Bhd.
03-Chem F4 (3P).indd 63 12/9/2011 5:57:55 PM