Page 5 - 1202 Question Bank Chemistry Form 4 KSSM
P. 5
MUST
KNOW Important Diagrams
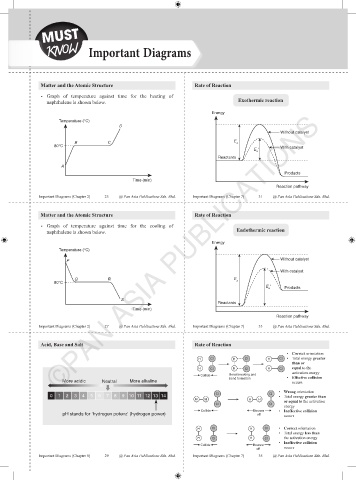
Matter and the Atomic Structure Rate of Reaction
• Graph of temperature against time for the heating of
naphthalene is shown below. Exothermic reaction
Energy
©PAN ASIA PUBLICATIONS
Temperature (°C)
D
Without catalyst
B C E a
80°C With catalyst
E '
a
Reactants
A
Products
Time (min)
Reaction pathway
Important Diagrams (Chapter 2) 25 @ Pan Asia Publications Sdn. Bhd. Important Diagrams (Chapter 7) 31 @ Pan Asia Publications Sdn. Bhd.
Matter and the Atomic Structure Rate of Reaction
• Graph of temperature against time for the cooling of
naphthalene is shown below. Endothermic reaction
Energy
Temperature (°C)
Without catalyst
P
With catalyst
Q R E
80°C a
E ' Products
a
S
Reactants
Time (min)
Reaction pathway
Important Diagrams (Chapter 2) 27 @ Pan Asia Publications Sdn. Bhd. Important Diagrams (Chapter 7) 33 @ Pan Asia Publications Sdn. Bhd.
Acid, Base and Salt Rate of Reaction
• Correct orientation
• Total energy greater
H Cl H Cl H Cl ● Correct orientation
than or
● Total energy greater than or
equal to the activation energy
equal to the
H Cl H Cl H Cl
activation energy
● Effective collision occurs
Collide Bond breaking and
bond formation • Effective collision
More acidic Neutral More alkaline occurs
• Wrong orientation
Cl Cl ● Wrong orientation
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 • Total energy greater than
● Total energy greater than or
H H H H or equal to the activation
Cl Cl equal to the activation energy
energy
● Ineffective collision occurs
Collide Bounce • Ineffective collision
pH stands for 'hydrogen potenz' (hydrogen power) off occurs
● Correct orientation
H Cl H Cl • Correct orientation
• Total energy less than
● Total energy less than
the activation energy
H Cl H Cl the activation energy
● Ineffective collision occurs
• Ineffective collision
Collide Bounce
off occurs
Important Diagrams (Chapter 6) 29 @ Pan Asia Publications Sdn. Bhd. Important Diagrams (Chapter 7) 35 @ Pan Asia Publications Sdn. Bhd.