Page 284 - Basic Principles of Textile Coloration
P. 284
Adsorbed dye/mmol kg–1 DYEING NYLON WITH ACID DYES 273
sites in the nylon. It will even displace any blue dye already adsorbed by the nylon.
Many pairs of dyes exhibit this type of competition behaviour.
For milling dyes, which have higher substantivity for nylon, the isotherms
showing the amount of dye adsorbed at equilibrium as a function of pH are similar
to those for simple acid dyes, but displaced to higher pH values and with
considerable dye adsorption above pH 7. Figure 13.9 shows an example.
As in the case of wool dyeing, the ion exchange mechanism is simple and
useful. It does not, however, explain the pronounced substantivity of milling and
metal-complex acid dyes in neutral solution, under conditions where the nylon
only has very few ammonium ion groups. In the case of these types of acid dye, the
dye–fibre interaction must involve forces other than the attraction of oppositely
charged ions. Obviously, dipole–dipole and hydrophobic interactions between the
dye and nylon molecules play an important role in determining the high
substantivity and good washing fastness of these types of dyes.
Many of the high substantivity acid dyes, particularly hydrophobic
monosulphonated or non-polar pre-metallised dyes, often dye nylon in amounts
well over the amino group content, even at pH values as high as 6–7. Build up of
deep shades is not a problem with such dyes. This phenomenon is called over-
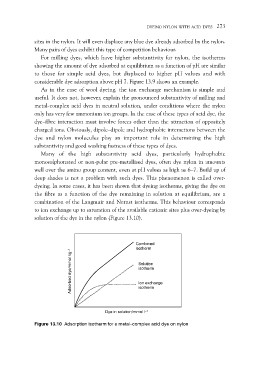
dyeing. In some cases, it has been shown that dyeing isotherms, giving the dye on
the fibre as a function of the dye remaining in solution at equilibrium, are a
combination of the Langmuir and Nernst isotherms. This behaviour corresponds
to ion exchange up to saturation of the available cationic sites plus over-dyeing by
solution of the dye in the nylon (Figure 13.10).
Combined
isotherm
Solution
isotherm
Ion exchange
isotherm
Dye in solution/mmol l–1
Figure 13.10 Adsorption isotherm for a metal–complex acid dye on nylon