Page 307 - Basic Principles of Textile Coloration
P. 307
296 DYEING CELLULOSIC FIBRES WITH DIRECT DYES
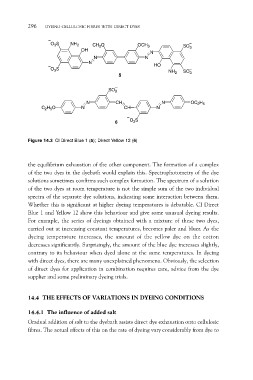
O3S NH2 CH3O OCH3 SO3
O3S N NH2 SO3
OH
N
N
N HO
5
C2H5O SO3 N OC2H5
N
N CH
N CH
6 O3S
Figure 14.3 CI Direct Blue 1 (5); Direct Yellow 12 (6)
the equilibrium exhaustion of the other component. The formation of a complex
of the two dyes in the dyebath would explain this. Spectrophotometry of the dye
solutions sometimes confirms such complex formation. The spectrum of a solution
of the two dyes at room temperature is not the simple sum of the two individual
spectra of the separate dye solutions, indicating some interaction between them.
Whether this is significant at higher dyeing temperatures is debatable. CI Direct
Blue 1 and Yellow 12 show this behaviour and give some unusual dyeing results.
For example, the series of dyeings obtained with a mixture of these two dyes,
carried out at increasing constant temperatures, becomes paler and bluer. As the
dyeing temperature increases, the amount of the yellow dye on the cotton
decreases significantly. Surprisingly, the amount of the blue dye increases slightly,
contrary to its behaviour when dyed alone at the same temperatures. In dyeing
with direct dyes, there are many unexplained phenomena. Obviously, the selection
of direct dyes for application in combination requires care, advice from the dye
supplier and some preliminary dyeing trials.
14.4 THE EFFECTS OF VARIATIONS IN DYEING CONDITIONS
14.4.1 The influence of added salt
Gradual addition of salt to the dyebath assists direct dye exhaustion onto cellulosic
fibres. The actual effects of this on the rate of dyeing vary considerably from dye to